- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
In vivo Recordings/Whole-cell Patch-clamp Recordings
Brain slice preparations have enabled investigators to make intracellular/whole-cell patch recordings from neurons while applying pharmacological agents but have a disadvantage in that the normal flow of information in neural circuits is disrupted. To test the acute effect of a compound on an individual neuron or the effect of pre-treatment with a drug on the spontaneous firing activity and firing rate of multiple neurons, Creative Bioarray performs a variety of in vivo single-cell recording studies.
Neurons from various parts of the brain can be recorded, including:
Dopamine (substantia nigra and ventral tegmental area)
Histamine (tuberomammillary nucleus)
Norepinephrine (locus coeruleus)
Glutamate pyramidal neurons (prefrontal cortex/hippocampus)
Serotonin (medial raphe nucleus and dorsal raphe nucleus)
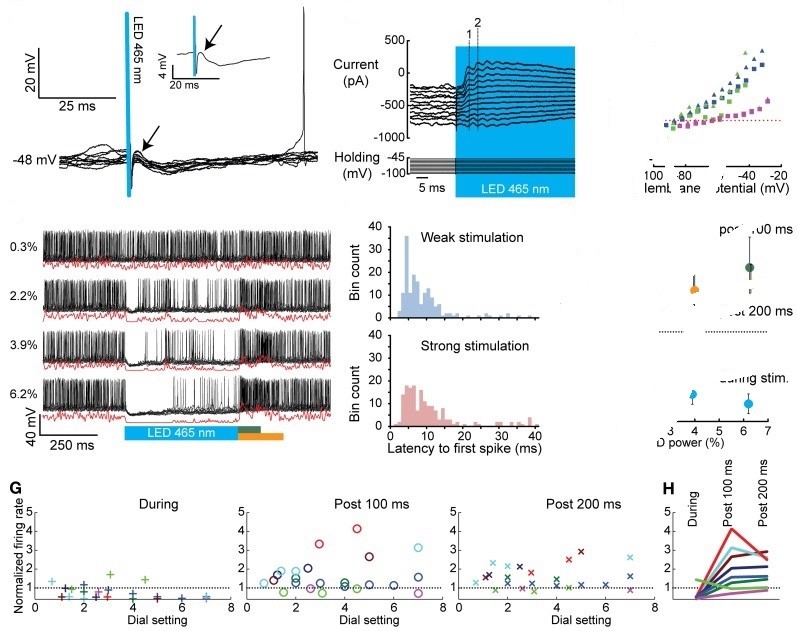
Fig. 1 Whole cell and extracellular in vivo recordings of CNs before, during, and after optogenetic activation of PCs
Multiple Recording Models
In vivo single unit recordings can be done either intracellularly or extracellularly. While extracellular recordings can only give spike information, intracellular single unit recordings can give information on sub-threshold information, resting potentials and postsynaptic potentials. The use of either technique depends on the specific application and what information is desired.
Intracellular
Intracellular single unit recordings provide much more information on single neuron discharges. They can give information on steady and resting membrane voltage, postsynaptic potentials, and spikes (action potentials) from both the axon and cell body.
Extracellular
Extracellular single unit recordings are more suitable for measuring extracellular action potentials. Extracellular recordings can easily measure spike discharge from a neuron with any suitably small electrode. This makes it much easier to obtain these signals in an awake and moving animals.
Combined recordings
This involves careful placement of extracellular and intracellular electrodes in a single neuron. The primary use for this is to provide a better understanding of the relationship between intracellular action potentials and extracellular spike recordings.
Reference
Witter L, et al. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Frontiers in neural circuits. 2013, 7: 133.
Related Section
Inquiry

