- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Cardiomyocyte Functions
During the discovery and launch of new drugs, those with undetected cardiotoxic side effects could have hazardous consequences and trigger lethal cardiac dysrhythmias (ventricular arrhythmias or TdP) in patients. It is therefore important to assess the potential cardiotoxic effects of new chemical entities (NCEs) at an early stage of drug development. Unfortunately, many of these compounds are not identified until they are in clinical trials after millions of dollars and years of effort have been invested. The late detection of cardiotoxic side effects (such as electrocardiogram QT-interval prolongation) induced by compounds of pharmacological interest, can dramatically impede drug research progress.
Therefore, for the sake of saving time and money, it is critical to assess the preclinical cardiac safety of these investigational products before entering clinical trials. Creative Bioarray provides series in vitro electrophysiology services for preclinical cardiac safety evaluations.
In vitro pharmaceutical profiling which can provide crucial information to estimate drug candidates with potential toxicity early in drug discovery is needed. An additional ICH guideline indicates that cardiac action potential duration (APD) in mammalian Purkinje Fibres, cardiac HERG-K+ channel interactions and in vivo ECG (QTc-intervals) measurements in conscious unrestrained animals (via Telemetry) should be conducted prior to first administration to humans. In vitro assays allowing precise control of drug concentration, at levels not easily attainable in vivo and at precise control of pacing rates, form a necessary adjunct to safety testing. Such design is provided by Purkinje Fibres which forms a multi-cellular tissue that propagates the AP into the muscular walls of the ventricle which is highly sensitive to drugs that produce LQT.
Fig.1 Drug Discovery Process
Early in the drug development, a variety of CVS in vitro electrophysiology services aimed at identifying this potential CVS toxicity are available in Creative Bioarray. These in vitro test systems have become powerful tools for predicting the proarrhythmic potential of drugs prior to their entry into the clinic. However, they should not only enhance the speed and efficiency at which drugs will be developed in the future, but also complement the in vivo preclinical cardiovascular evaluation. Early testing for multiple effects on key cardiac ion channels fully resolves discordances and prevents advancement of cardiotoxic drug candidates. From single cell to isolated tissue, our large-scale range of in vitro services includes conventional manual and automated (Rapid ICE) Ion Channel Electrophysiology and organ preparations. All CVS electrophysiology services are maintained and enabled by comprehensive resource and our expert staff.
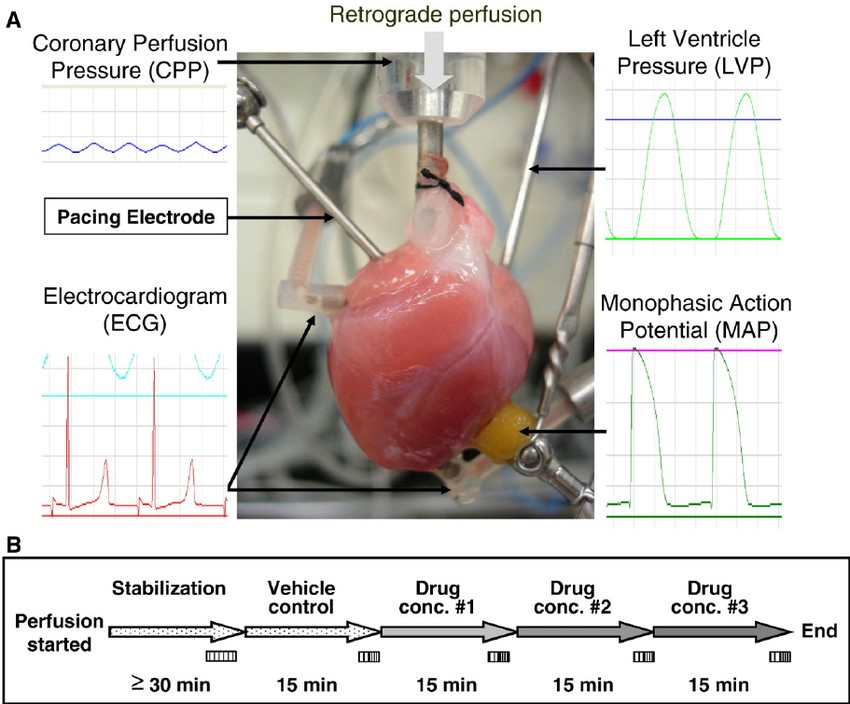
Fig. 2 The isolated heart and experimental protocol
Although the above-described ionic assays (conducted on isolated cells) benefit from incredible resolution, they lack the integrated nature of a spontaneously-beating heart. With less resolution, but greater physiological context, the isolated heart assay uncovers the impact of the compound on the ionic currents and mechanical phenomena which underlie the heart's functions: electrical conduction and mechanical contraction. Creative Bioarray provides Langendorff perfused heart ECG and contractility analysis to test drugs on isolated animal hearts. We can identify effects of your compounds on the ECG (QT interval, QRS), heart contractility (LVDP, dP/dt) and coronary flow. At least three hearts will be used per concentration. Final reports include drug effects on the study parameters containing a results summary, methods description, quality standard statement and any amendments or deviations included as an appendix.
Highlights
Data are generated under physiological conditions which is essential for the pre-clinical characterization of NCEs cardiotoxic effects.
3D structure of cardiac cell models can be more representative of in vivo tissue than traditional monolayer cell culture.
Functional Tests
Multicellular Conduction
Physiological Tests on Isolated Cardiomyocyte
Quotation and Ordering
If you have any special needs in our Cardiomyocyte Functions Service, please contact us at Email or Telephone for this special service. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
Reference
Guo, L., et al. Validation of a guinea pig Langendorff heart model for assessing potential cardiovascular liability of drug candidates. J Pharmacol Toxicol Methods. 2009; 60: 130-151
Related Section
Inquiry

