- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Primary Cultured Neurons
Cultured neuronal cells are important parts of neuroscience laboratories. The ability to experiment on real neurons in a controlled environment under specific conditions is essential to understand cellular and molecular mechanisms in the brain during development and in disease.
Primary neuronal and glial cultures isolated from rodent brains are widely used to study brain function in a controlled in vitro environment. Human fetal brain tissue can be used to generate neuronal, microglia, and astrocyte cultures. The neuronal cultures from human fetal brain tissue help to understand development of neurons. However, certain experimental procedures need human neuronal and glial cells to confirm the cellular functions. Because the human brain is the least accessible organ, experimental studies on brain cell cultures provide valuable information. Isolation and culture of neurons are tricky but very important techniques in neuroscience research. These cells are very fragile, and primary culture of brain cells can be challenging.
Creative Bioarray provides a comprehensive service for the isolation and enrichment of different cell types from human brain tissue, and the optimal conditions needed to achieve viable and healthy neuronal cultures.
Studies showed that there were differences in N-methyl-d-aspartate (NMDA) and non-NMDA receptors expression pattern and vulnerability to excitotoxicity in culture with respect to the expression of the receptors between human and rodent neurons in culture. Such differences noticed between highly evolved human brain and laboratory animal models exhibit concerns with extrapolating discoveries in rodents to human brain development and disease. This underscores the importance of studying cellular mechanisms and disease processes using human brain cells.
Brain development has primarily been studied in invertebrates or rodents. Distinct pattern of gene expression was detected in human fetal cortical areas suggesting evolutionary patterns underlying human-specific neural traits. Human-specific histone methylation signatures and epigenetic regulation were also observed using human fetal cortical neurons, which could play an important role in the emergence of human-specific gene expression networks in the brain. Research on human brain cells will help to better understand disease processes observed in small animal models.
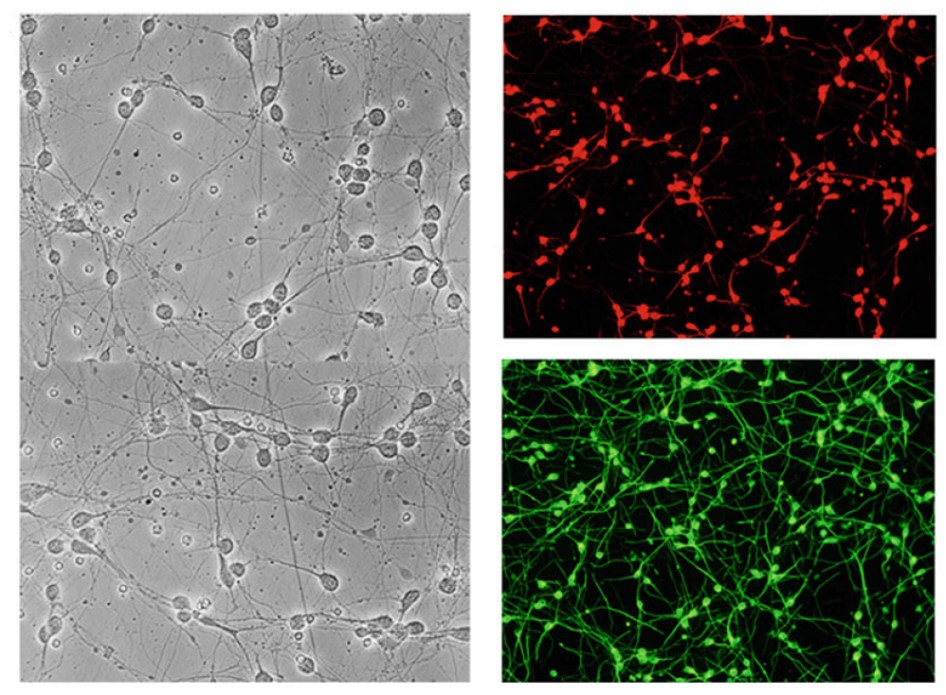
Fig.1 Neuronal cells in culture under bright field and stained with MAP-2 or β-III tubulin
Processes involved in several neurodegenerative diseases such as traumatic brain injury, Alzheimer's, Parkinson's, and Huntington's diseases, and amyotrophic lateral sclerosis (ALS) are poorly understood. Neurons in several brain regions are severely affected in these neurodegenerative disorders, with loss in synapse and compromised neural networks. In vitro studies with human neurons play an important role in understanding causes for these diseases and to test new therapeutic approaches. It is important to develop experimental conditions representing disease at the cellular level to understand the function of a single cell type in brain development and disease.
Neuronal cultures from cells isolated from human fetal tissue contain cells in various stages of development. They readily form neuritis in culture and establish synaptic connections. Axons of human fetal neurons grow slowly compared to rodent cells, and the human neuron cultures can be maintained for relatively longer duration up to 1 month. Creative Bioarray provides neurons isolated from the human brain which are cryopreserved at primary cultures and delivered frozen. Each vial contains >1x10^6 cells in 1 ml volume. The cells are characterized by immunofluorescent method with antibodies to neurofilament, MAP2, and beta-tubulin III and the cells are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. Human neurons are guaranteed to further culture in the conditions provided by Creative Bioarray.
Creative Bioarray also provides cocultures of neurons and glial cells which can be maintained in a controlled environment to follow the cross talk between different cell types. For coculturing purposes, neurons and glial cells obtained from the same donor brain tissue are preferred. Human neurons, astrocytes, oligodendrocytes, and microglia can be isolated successfully from the same fetal tissue. Neuronal and glial cells are used to study disease mechanisms involved in several neurodegenerative diseases, such as HAND, AD, PD, multiple sclerosis, stroke, and ALS. Either neurons or glia can be treated with amyloid-beta peptide (Aβ), which forms insoluble plaques in the brain. Aβ when added to fetal neurons induces excitotoxic cell injury and death.
Reference
Xiong, H., & Gendelman, H. E. (Eds.). Current laboratory methods in neuroscience research. Springer. 2014
Related Section
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
Inquiry

