- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Ion Flux Assay
When a concentration differential exists for an ion across a cell membrane and an open channel of appropriate specificity exists on that membrane, ions may pass through the channel down their electrochemical gradient resulting in ion flux. These ion fluxes have been used for decades as a means to measure the activity of ion channels.
Ion flux assays are cell-based functional assays. They trace ionic flux through channels by radioisotopes, or detect ionic flux using highly sensitive flame atomic absorption spectrometers (AAS) or using fluorescence ion-specific probes/biosensors. Ion flux assays use a radioactive species in combination with a scintillation counter or use a tracer ion measured by atomic absorption/emission spectrometry.
In the 1980's the development of ion-selective fluorescent dye-based indicators revolutionized the measurement of ion flux. These indicators improved the ease of use, as well as spatial and temporal resolution of ion flux measurements. More recently, the use of automated flame photometry-based ion flux assays has been proven effective as an alternative to fluorescent and radionuclide-based techniques for some ion channel assays.
Here, in Creative Bioarray, if you are doing target channel screening, we can provide a validated ion flux assay fit for your purpose.
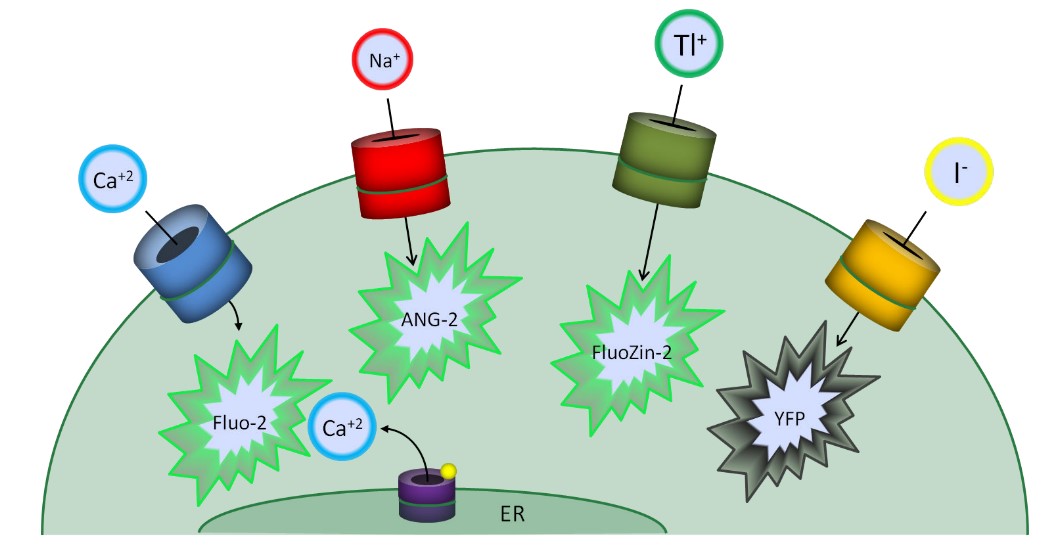
Fig.1 Fluorescence-based Ion Flux Assay Formats
Applications
Measure the flux of physiological ions (e.g. calcium, sodium, magnesium, zinc, and protons, etc.).
Measure the flux of surrogate ions (e.g. thallium, cobalt, and iodide). For high-throughput compatible assays, potassium congener thallium is used, changing the fluorescence of FluoZin-2. For chloride channels, the use of surrogate anions (e.g. iodide) via engineered yellow fluorescent protein-based anion sensors.
Measurement of intracellular calcium flux mediated through the IP3 receptor downstream of Gq-couple seven transmembrane receptors (aka GPCRs).
Reference
McManus O B., et al. Ion channel screening. 2012.
Related Section
Inquiry

