- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Cardiac Ion Channel Screen Panels
The drug-induced cardiovascular complications restrict the use of many successful clinical treatments such as anticancer therapies. The majority drug-induced cardiovascular complications include overt cardiac dysfunction as well as some negative impact such as hypertension, ischemia, and arrhythmias. Enormous efforts and investments have been made throughout the process of new drugs development to identify adverse effects of cardiovascular systems and minimize the risks for patients. Approximately one third of compounds in pre-clinical development exhibit some levels of cardiac risks.
One of the most important cardiovascular (CVS) liabilities in safety pharmacology is the life-threatening condition known as Torsades de pointes (TdP) which is principally mediated by changes in the activity of the hERG (Kv11.1) channel and other important cardiac ion channels (Cav1.2, Nav1.5, Kv4.3, Kir2.1 and Kv7.1). Many compounds can affect the activities of the ion channels or transporters, thus have the potential of delaying the ventricular repolarization and leading to prolonged QT interval.
Voltage gated ion channels are central in defining the fundamental properties of the ventricular cardiac action potential (AP), and are also involved in the development of drug-induced arrhythmias. Many drugs can inhibit cardiac ion currents, including the Na+ current (INa), L-type Ca2+ current (Ica-L), and K+ currents (Ito,IK1,IKs, and IKr), and thereby affect AP properties in a manner that can trigger or sustain cardiac arrhythmias. From 1997 to now, the retraction ratio from market increased from 5.1% to 33.3% because of cardiac toxicity. During the early stages, taking cardio-safety evaluation will help reducing time and money cost in drug discovery.
Staffed with a group of experts that have gained years of experience in ion channel safety assays and cardiotoxicity assessment, Creative Bioarray provides electrophysiological services of in vitro (such as hERG safety) assays and in vivo (such as QT assay by ECG) analysis.
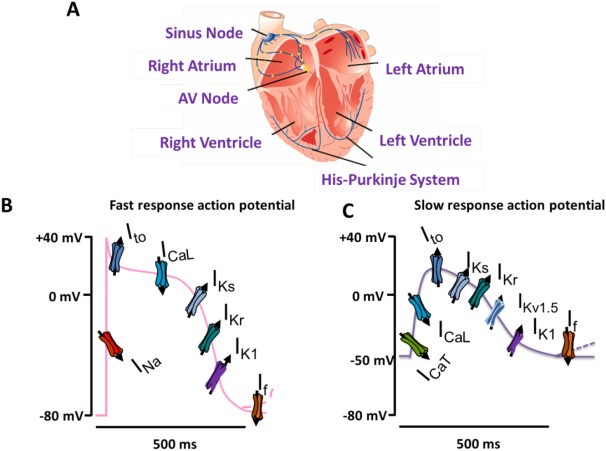
Fig. 1 The cardiac electrical conduction system and genesis of the cardiac action potential in fast-response and slow-response tissues.
Creative Bioarray has developed a GLP-compliant, in vitro electrophysiological service which offers clients a robust ion channel platform from which to characterize the mechanism of action and safety of a lead compound. This in vitro cardiac profile screen provides a comprehensive safety analysis including inhibition tests and IC50 metrics.
The risk of approving drugs with the potential to produce TdP in patients has effectively been eliminated by evaluating their ability to block the hERG channel in vitro and prolong ventricular repolarization and the QTc interval of the ECG. With conventional patch-clamp and MEA technology, Creative Bioarray can provide services to detect and analyze effects of candidate drugs on key cardiac ion channels and pathways, with predictive and reproducible data.
Creative Bioarray now offers a cardiac safety panel assays. We perform tests to identify drug interactions with cardiac ion channels that act as target for QT prolongation. Our cardiac ion channel panel can help minimize unnecessary triage of promising compounds.
Highlights
- hERG safety assay in accordance with the ICH S7B guideline
- Many other ion channels inhibition assays are also available
- Automated QPatch-HT system with better consistency at a lower cost
- Conventional whole cell patch clamp assay is also available
- CHO-hERG cells or HEK293-hERG cells
Ion Channels Test Panels
Quotation and Ordering
If you have any special needs in our Cardiac Ion Channel Screen Service, please contact us at Email or Telephone for this special service. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
Reference
- Huang H., et al. Cardiac voltage-gated ion channels in safety pharmacology: Review of the landscape leading to the CiPA initiative. J Pharmacol Toxicol Methods. 2017; 87: 11–23.
Related Section
Inquiry

