- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
hERG (Ikr, Kv11.1)
The Kv11.1 channel, a potassium ion channel encoded by the human ether-à-go-go-related (hERG) gene (thus also referred to as hERG channel), plays a very critical role in the cardiac action potential repolarization (phase 3). Inhibition of hERG function causes lengthening of ventricular action potential duration and is the most common mechanism of QT interval prolongation. Therefore, evaluating effects of compounds on hERG activity early in drug discovery can greatly reduce the risk of putting extensive efforts in cardiotoxic drugs and prevent them from entering the market. ICH S7B guideline regulates in vitro IKr assay (such as hERG safety assay) and in vitro QT assay for identifying and assessing the potential of a test compound to delay ventricular repolarization.
Staffed with a group of experts that have gained years of experience in ion channel safety assays and cardiotoxicity assessment, Creative Bioarray offers hERG safety assay in accordance with the ICH S7B guideline to evaluate compound cardiovascular safety and to support drug development.
hERG Inhibition assays (screens or GLP-compliant, pre-IND assays) offer extraordinary sensitivity, much greater than whole-organ assays, or whole-animal assays. Of course, proper study design and data interpretation decreases the risk of false positives resulting from this immense sensitivity. It is therefore imperative, when considering hERG inhibition patch-clamp data, to obtain expert interpretation, usually from the study director who oversaw the study.
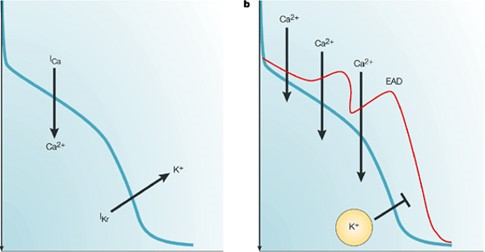
Fig. 1 The mechanism of delayed repolarization caused by IKr inhibition. The balance between inward and outward currents determines the morphology and duration of the action potential, and consequently the duration of the QT interval. Drug-induced inhibition of the IKr current can delay repolarization, and prolong the action potential duration and the QT interval.
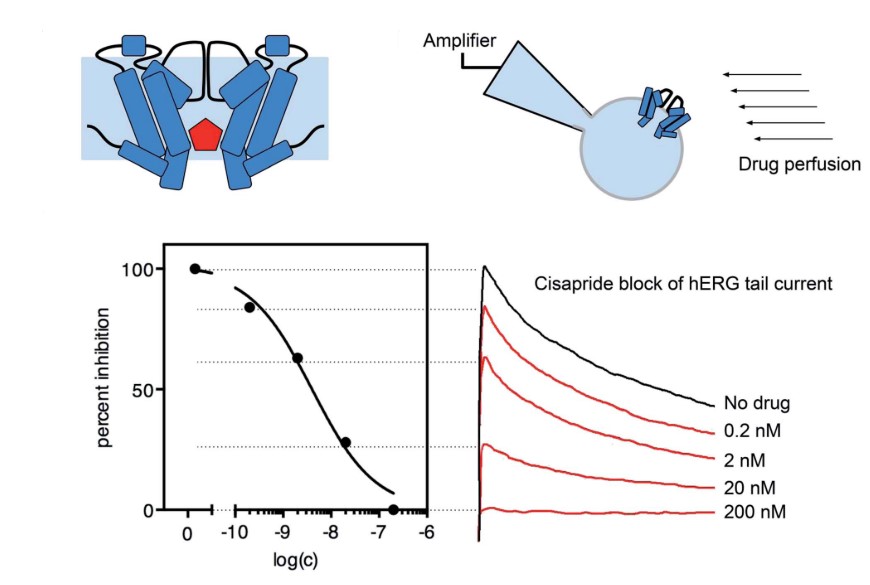
Fig.2 Drug block of hERG channel current
Creative Bioarray uses the state-of-the-art automated QPatch-HT system to provide a higher-throughput hERG safety assay with better consistency at a lower cost. For small number of compounds or compounds identified with QPatch, we can also perform conventional whole cell patch clamp assay to get detailed mechanistic information. Our GLP level hERG safety assay can provide high quality data for registration purposes.
Highlights
- Cell Lines: CHO-hERG cells; HEK293-hERG cells
- Method: Electrophysiology (manual or automatic)
- Protocols include exploratory non-GLP screening of single high concentration or IC50
- Test Compound Concentration: As required by the client
- Number of Replicates: As required by the client
- Vehicle control: Included
- Cumulative curves: Included
- QC Seal resistance > 50 MOhms
- Pre-compound current ≥ 0.1 nA
- Deliverables: IC50; Report
- Both GLP-compliant and non-GLP, faster study design available
- Conducted at physiological temperature
- Analysis of both amplitude and current kinetics as indicators of inhibition
- FDA-ready hard copy and e-report for electronic IND submission
- Part of the core battery of Safety Pharmacology tests required for IND submission
Advantages
- Customer focused approach, beyond your expectations
- Scientific excellence in in vitro pharmacology and a technical team with the same high standards
- A quality service
- A flexible service- we support a cross-section of global clients, from small biotech to large pharmaceutical companies, and academia, providing both validated assays and tailored protocols.
Creative Bioarray provides manual and auto patch clamp functional hERG assay to evaluate drug's inhibition or potentiation effect on hERG channel. In this assay, CHO-hERG cells or HEK293-hERG cells are used to specifically assess the effect of test compounds on the hERG channel. Pre-compound current and post-compound current are measured by patch clamp, and applied to the calculation of hERG inhibition.
Creative Bioarray's experienced technicians allow us to provide customized assays. We are capable of adjusting the assays according to the detailed requirements of our clients. If you have any special requirements or related questions, please do not hesitate to contact us. Our experts will do their best to assist you.
References
- Food, U. S. "Drug Administration Guidance for industry: ICH S7B nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals." Federal Register. 2005;70: 61133-61134.
- Kratz JM, et al. Natural products modulating the hERG channel: Heartaches and hope. Nat Prod Rep. 2017; 34: 957–980.
Related Section
Inquiry

