- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Acute/Chronic Heart Failure Model
Heart failure (HF) is a leading cause of morbidity and mortality in the United States (about 5 million people in the U.S. have heart failure). HF diminishes quality of life and it contributes to 300,000 deaths each year. Despite a number of important therapeutic advances for the treatment of symptomatic HF, the prevalence, mortality, and cost associated with HF continue to grow in the United States and other developed countries.
The treatment of HF is a major challenge for drug and medical device development. Since the causes of HF are myriad, impaired filling and/or ejection of blood induced decreased ability of the heart to provide sufficient cardiac output to support the normal functions. There is a great need in developing novel preventative and reparative therapies as current treatments primarily focus on slowing the progression of this syndrome. The development of novel HF therapies requires testing of the putative therapeutic strategies in appropriate HF animal models.
Animal models of Acute/Chronic HF have many advantages: relatively inexpensive: a large sample size can be produced in a relatively short period of time; it can be used to study long-term pharmacological interventions including long-term survival studies.
Creative Bioarray provides multiple heat failure models for customers to choose:
Acute Heart Failure Models
Heart failure induced by acute coronary occlusion
Heart failure induced by acute coronary occlusion and reperfusion
Heart failure induced by cardiotoxic agents, e.g. doxorubicin
Chronic Heart Failure Models
Heart failure induced by permanent chronic coronary occlusion
Heart failure induced by coronary occlusion and reperfusion-induced infarction
Heart failure induced by cardiotoxic agents, e.g. chronic low-dose doxorubicin treatment
Isoproterenol-induced heart failure model
Salt and volume overload-induced heart failure
Transverse aortic constriction-induced heart failure
Chronic pacing-induced heart failure
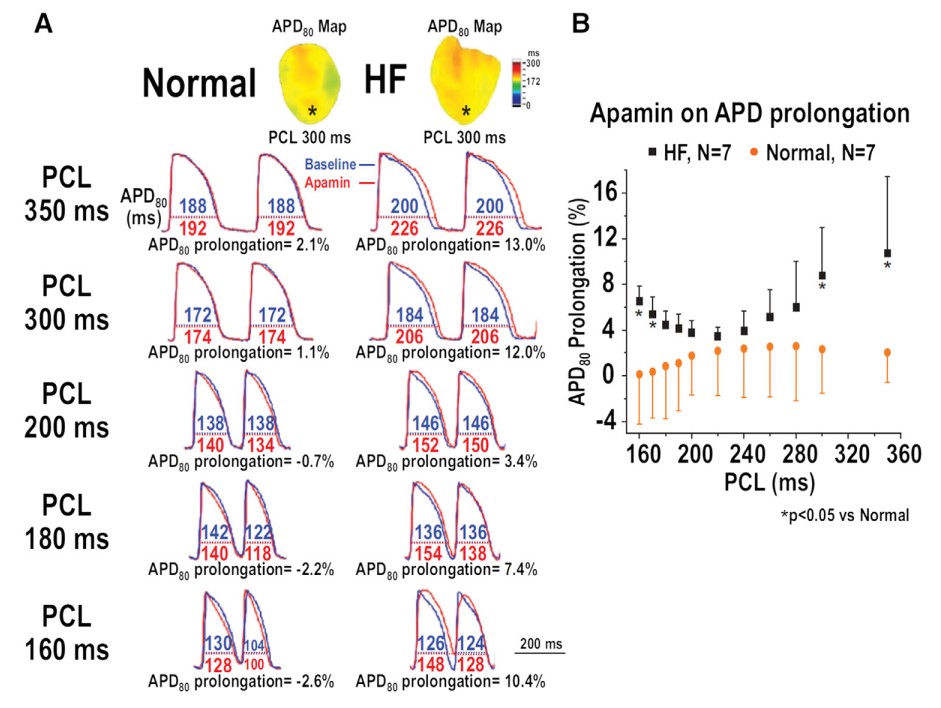
Fig.1 Effect of apamin on the percentage of action potential duration (APD) prolongation in normal and failing rabbit ventricles
Multiple indicators are analyzed for confirming the success of HF animal model establishing:
High blood pressure
Breathlessness at rest or with minimal activity
Fatigue Edema
Left Ventricular hyper-trophy (concentric hypertrophy with cardiac myocytes demonstrating an increased diameter) or increased LV mass
Impaired active relaxation Impaired passive filling
Impaired Ejection Fraction (late)
Dilated Left Ventricular internal chamber (late)
Enlarged Left Atrium
Myocardial fibrosis (reactive and replacement)
Abnormal pressure/volume relationship in the LV with increased LV filling pressure (increased chamber stiffness) relative to LV volume
Decreased intra-myocardial capillary density
Coronary arteriolar thickening
Decreased coronary blood flow reserve Increase biomarkers such as NT-proBNP, BNP, or troponin
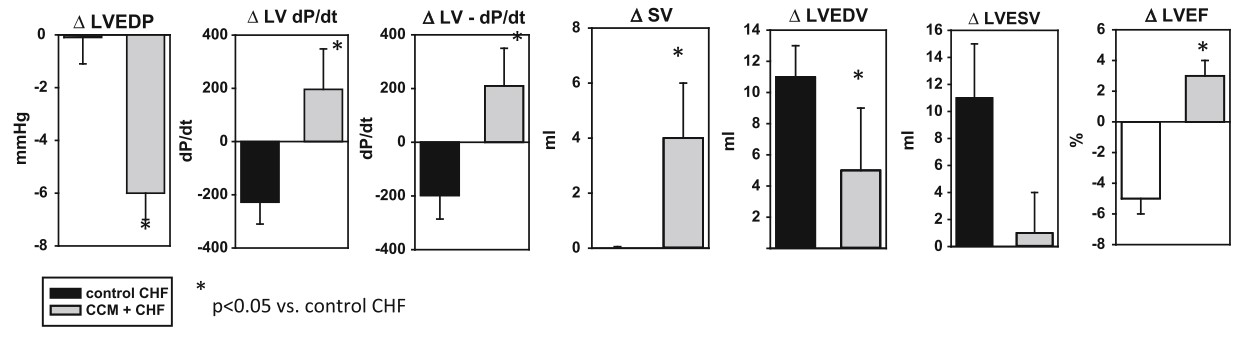
Fig. 2 Chronic effect of CCM on cardiac structure and function in a canine model of HF
Creative Bioarray also provides transgenic animal models of hypertrophy and heart failure which are critically important for understanding the molecular alterations underlying the development of the disease. Addition or deletion of genes in transgenic mice together with miniaturized physiological techniques to evaluate the resulting cardiac phenotypes allow the identification of genes that are causative for heart failure and to evaluate molecular mechanisms responsible for the development and progression of the disease. Hypertrophy and heart failure models in mice will be used in the future to study rescue or repair by knockout or overexpression of specific genes.
Creative Bioarray provides animal models of HF which mimic distinct features of human HF to the study of the consequences of gene transfer and molecular techniques to correct disturbed subcellular processes in the failing heart. These kinds of studies are a prerequisite to the development and introduction of molecular strategies for the treatment of HF in patients.
References
Houser SR, et al. Animal models of heart failure a scientific statement from the American Heart Association. Circ Res. 2012; 111: 131–150.
Morita H, et al. Long-term effects of non-excitatory cardiac contractility modulation electric signals on the progression of heart failure in dogs. Eur J Heart Fail. 2004; 6:145–150.
Chang PC, Chen PS. SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc Med. 2015; 25: 508–514.
Related Section
- Alzheimer's Disease Model
- Parkinson's Disease Model
- Huntington's Disease Model
- Epilepsy Model
- ALS Model
- Psychiatric Model
- Autism Spectrum Disorder
- Cerebral-Spinal Injury Model
- Pain Model
- Stroke Model
- Fragile X Model
Inquiry

