- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Acute Isolated/Cultured Cardiomyocytes
Isolated adult cardiac myocytes maintained in primary culture have been used as a model of the adult myocardium for more than 30 years. With the recent advances and current increasing interests in using molecular biological techniques to investigate cardiac physiology, culturing myocytes is becoming an increasingly important technique.
To study and understand the mechanisms underlying normal cardiovascular function, cardio-protection, and cardiovascular diseases, Creative Bioarray uses cultured primary cardiomyocyte which can be easily isolated and cultured displaying highly viable and functional properties for researches.
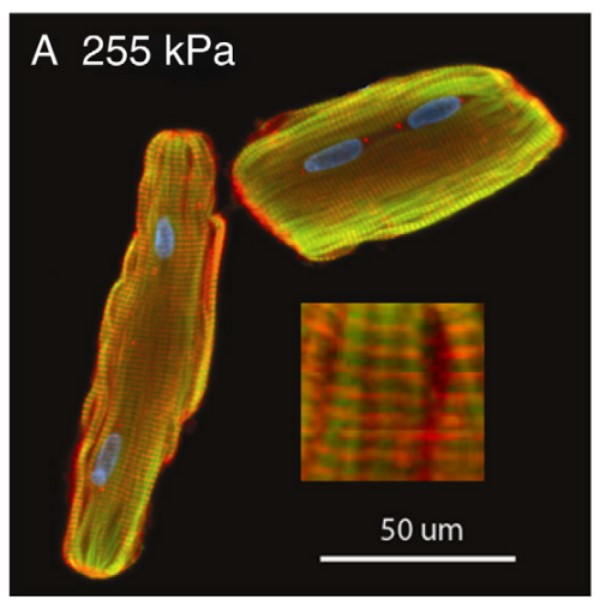
Fig. 1 Immunofluorescence images of z-disk organization in adult cardiomyocytes after 48-h
Advantages
Compared with whole heart, cardiomyocyte cultures are relatively pure, with limited contaminating cell types such as endothelial cells. Thus, cardiomyocyte cultures provide a homogeneous population of single cells, which are easy to visualize and manipulate. In addition, preparations of heart cells isolated from small mammals like mouse and rat enable a large number of quick, relatively low-cost experiments compared to studies conducted in whole animals.
Usage of isolated cardiac myocytes has a number of additional advantages such as the ability to select cells from different areas of the heart including the atria, left/right ventricle, the conductive system or a specific region of the heart following myocardial infarction. As well, while imaging techniques are often limited in thick tissue, isolated cells are well-suited for experiments aimed at visualizing cellular structure and the precise localization of intracellular molecules. Isolated cardiomyocytes are also routinely used for studies examining intracellular Ca2+ homeostasis, cellular mechanics, protein biochemistry and can be easily infected or transfected for gene transfer studies. Thus, isolated cardiomyocytes can be utilized in an array of experimental settings to provide valuable insight into cellular and sub-cellular physiology. By combining these data with experimental findings made in vivo and in intact tissue, animal models can be employed to examine cardiac function in a hierarchical manner.
While immortalized cardiac cell lines such as HL-1 cells are commercially available, acutely isolated cells are more physiologically relevant both structurally and functionally to the living organism. Cardiomyocyte isolation techniques are therefore of critical importance, as are methods for cell culture which aim to preserve as closely as possible the in vivo integrity and function of the cardiomyocytes.

Fig. 2 Typical alterations in cardiomyocyte morphology during culturing
During cardiomyocyte isolation, since the heart is a solid organ with strong intercellular attachments, which makes the dissociation process more difficult and time consuming. Thus, isolating primary cardiomyocytes from neonatal mouse or rat hearts has typically been a time-consuming, labor-intensive task. Isolating primary cardiomyocytes is a delicate process involving the controlled use of enzymes to disrupt complex protein and intercellular matrix interactions found in heart tissue. Creative Bioarray has screened several different proteases and collagenases individually and in combination to determine the most efficient digestion strategy.
The Action Potential Kinetics (APD) aka Purkinje Repolarisation assay can be conducted in cardiac Purkinke fibers, ventricular strips or ventricular papillary muscles. The shape of the action potentials and the complement of ionic currents which underline the action potentials is different in the various tissues, and proper selection of the tissues exposed ensures the best sensitivity for the assay. Creative Bioarray isolates cardiac Purkinke fibers, ventricular strips or ventricular papillary muscles according to customers' requirements.
Isolated cardiomyocytes from small rodents are an invaluable tool in the study of cellular level function and regulation of electrophysiology, intracellular calcium fluxes, contractile mechanics and protein expression. The ability to culture myocytes, even for short times, further benefits researchers by permitting the incorporation of transgenes into cells and facilitating longer-term treatments. Combined with the recent availability of transgenic animals, the array of techniques for manipulating cellular physiology in the investigation of molecular mechanisms regulating function has vastly expanded.
Although myocytes have been isolated and cultured for a number of years by standard techniques, it remains somewhat of an art to consistently produce high-quality cells. Creative Bioarray has knowledge of the described reagents and protocols, along with common caveats and critical points in the procedures will enable our customers to more easily establish cell-based research strategies, and advance the pursuit of therapeutics for human disease.
References
Loucha WE, et al. Methods in Cardiomyocyte Isolation, Culture, and Gene Transfer. J Mol Cell Cardiol. 2011; 51: 288–298.
Galie PA, et al. Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc Pathol. 2013; 22: 219–227.
Related Section
- Xenopus Oocyte Screening Model
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
Inquiry

