- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Optogenetics
Optogenetics is a biological technique to control or to monitor neural activity with light, which is achieved by genetic induction of light-sensitive proteins. It is a neuromodulation method that uses a combination of techniques from optics and genetics to control and monitor the activities of individual neurons in living tissue- even within freely moving animals and to precisely measure these manipulation effects in real-time.
Creative Bioarray applied optogenetics by combination of genetic and optical methods to achieve gain or loss of function of well-defined events in specific cells of living tissue. This method is based on the modulation of ion channel activity by light.
The key reagents used in optogenetics are light-sensitive proteins, for example, optogenetic activators like channelrhodopsin (ChR), halorhodopsin, and archaerhodopsin (Arch). While monitoring of neuronal activity can be performed with genetically encoded sensors for ions (e.g. GCaMP for calcium, synapto-pHluorin for vesicular release, GluSnFRs for neurotransmitter and Arc lightning or ASAP1 for membrane voltage). Optogenetics technique has the advantage of operating with high spatial and temporal resolution at multiple wavelengths and locations.
For specifically recording single neuronal activities, our optogenetics tools enable researchers to excite or inhibit specific neurons with unprecedented temporal and spatial resolutions using light. By recording the electrical activity surrounding a neuron that has been stimulated or inhibited, it is possible to establish a direct link between a neurons excitability and the consequences in associated cells.
Advantages
Specific localization / targeting
Choice of neuronal sub-population
High spatial and temporal resolution
Specific action
Multiple wavelengths
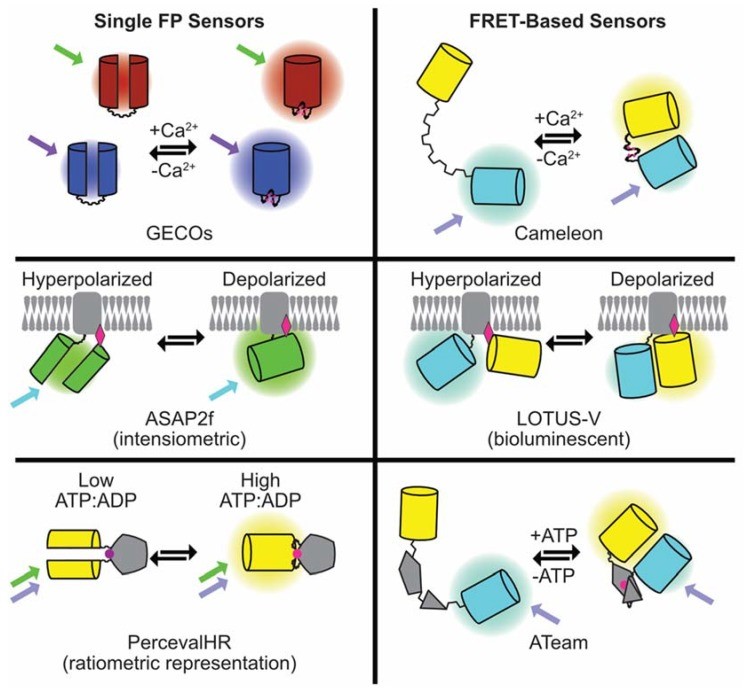
Fig. 1 Summary of the composition and mode of action of the genetically-encoded fluorescent sensors for Ca2+, voltage, and ATP discussed in the article.
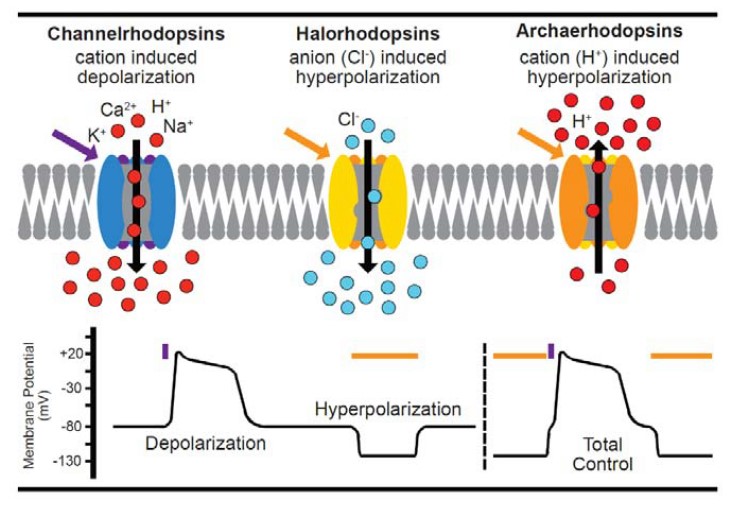
Fig. 2 Summary of optogenetic actuators
Activation: The channelrhodopsins-2 (ChR2) is a light-gated ion channel. ChR2 has broad cation conductance, including for Na+, K+, and even Ca2+.
Inhibition: The halorhodopsins (NpHR) is a light-activated ion pump transporting chloride. NpHR control gradients across the cell membrane by transporting chloride ions from the extracellular medium into the cell.
With specific wave lengths (470nm and 589nm), we are able to control which ions pass the membrane. Inserted in neurons, the control of the ChR2 or NpHR allows to activate or inhibit specifically the transfected cell population.
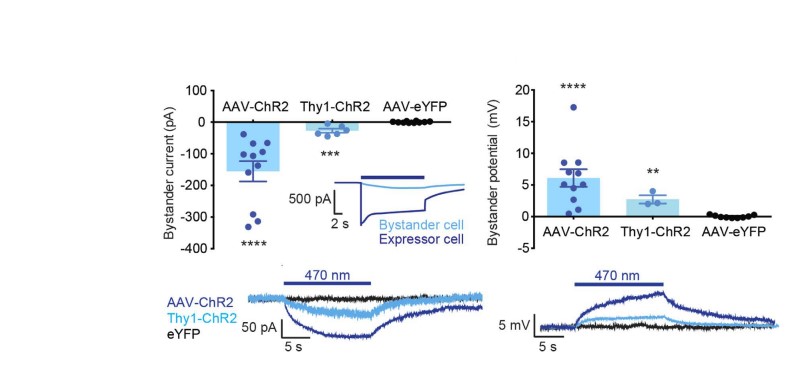
Fig.3 Depolarizing bystander currents for ChR2 hippocampal bystanders, Thy1-ChR2 cortical bystanders and YFP controls. Inset: comparison with ChR2 photocurrent.
With optogenetics, Creative Bioarray is able to study a specific neural network without affecting the operation of neighboring structures. Coupled with electrophysiological recordings, we perform in vitro and in vivo studies, that enable to observe the causal relationship between the activity of targeted brain pathways and behaviors of freely moving animals.
References
Ferenczi EA, et al. Optogenetic approaches addressing extracellular modulation of neural excitability. Sci Rep. 2016; 6: 1–20.
Broyles C, et al. Fluorescent, Bioluminescent, and Optogenetic Approaches to Study Excitable Physiology in the Single Cardiomyocyte. Cells. 2018; 7: 51.
Duebel J, et al. Optogenetics. Curr Opin Ophthalmol. 2015; 26: 226–232.
Related Section
- Neuronal Tract Tracing
- Manual Patch-clamp Technique
- Automated Patch-clamp
- Multi-Electrode Array (MEA)
- FluxOR™ Thallium Assay
- FLIPR Detection System
Inquiry

