- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Neuronal Tract Tracing
A major goal of neuroscience studies is to explore connections between specific types of neurons and the functions of these connections in the nervous system. Most of what we know today about the pathways and cross pathway connections of neurons has been discovered by using neuronal tract tracing techniques. Neuronal tract tracing technique was developed based on two principles, Wallerian degeneration and axon flow theory, and has been enhanced by knocking in an enhancer or inhibitor to a chain of neurons in studying specific pathways with molecular biological functionality.
In combination with stereotaxic injection of tracers, electrophysiology and behavior methods, neuronal tract tracing has made a great contribution to the understanding of brain function by unveiling dramatic neuroanatomical networks. Actually, this principle can be applied to mammals by stereotaxic injection of vectors carrying an enhancer or inhibitor gene without in-depth knowledge of the genetics of the targeted neuronal cell type in a pathway or a system.
Here in Creative Bioarray, we provide multiple neuronal tract tracing strategies:
Anterograde tract tracers: eg, HRP, Phaseolus vulgaris, BDA (Biotinylated dextran amine), CTB (Cholera toxin subunit B)
Retrograde tract tracers: eg, HRP
Transganglion tract tracer
Intracellular (anterograde and retrograde) tracer: eg, Biocytin, Neurobiotin.
The tract tracing, including anterograde, retrograde, and transganglion tract tracing, can be achieved through axon transportation of tracers. Based on the visibility of the tracers, they are classified as fluorescent and non-fluorescent tracers.
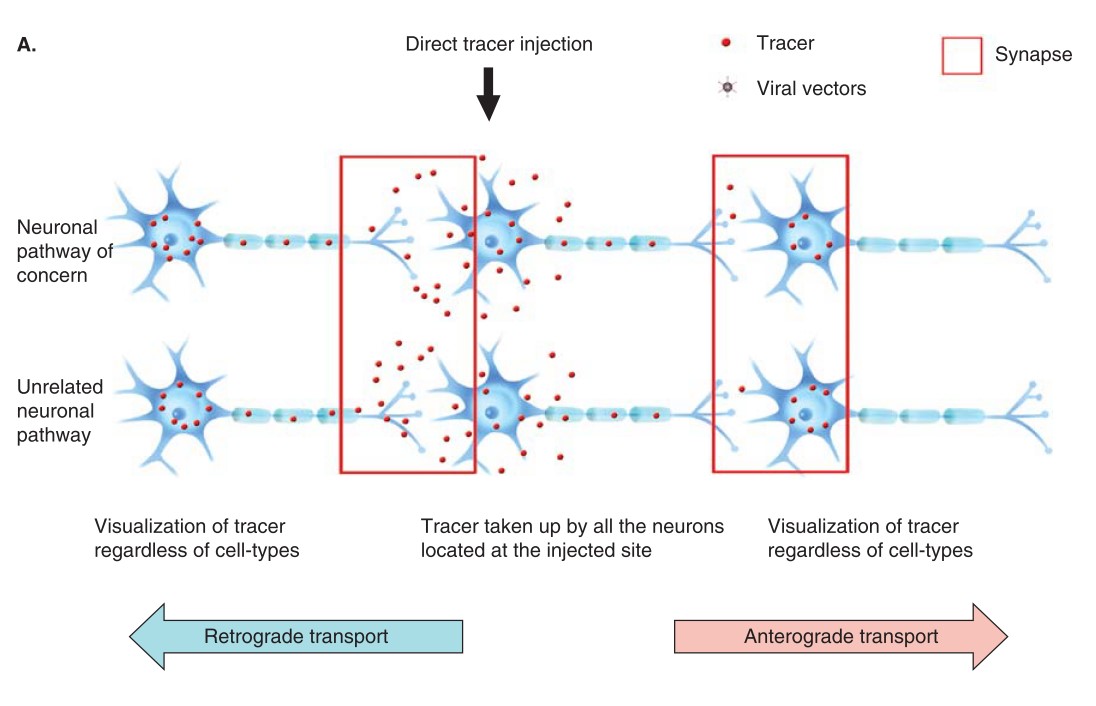
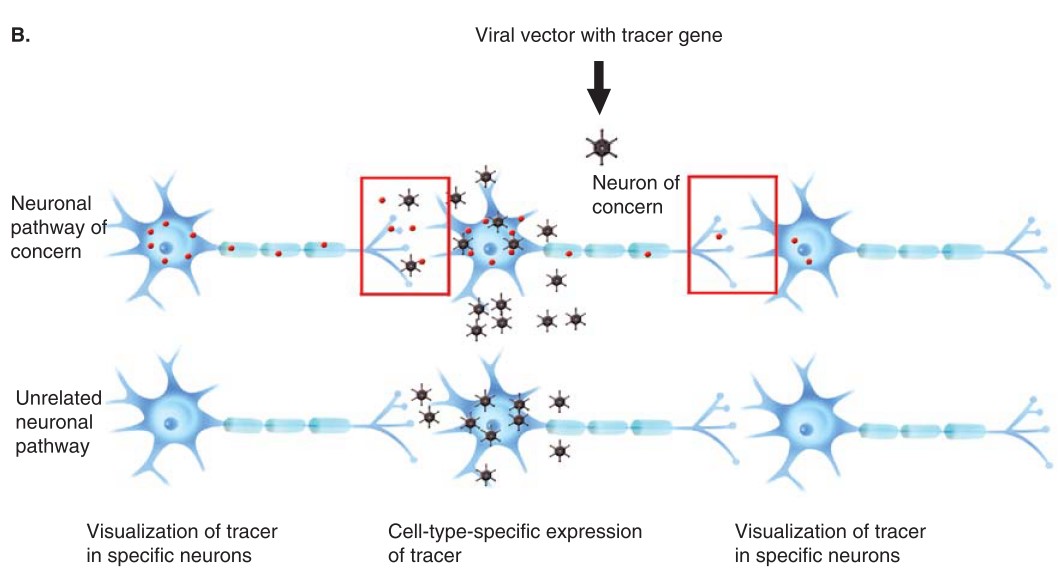
Fig. 1 Trans-neuronal transfer of tracer using conventional and viral tracer gene delivery
The most innovative tract tracing similar to “genetic dissection” in mammals is knock-in of a viral glycoprotein receptor gene into a specific group of neurons in central nervous system. This can be done by either delivery of a transfecting vectors or by using a receptor gene transgenic mouse and by injecting glycoprotein-coated neurotropic virus to the area containing that specific group of Neurons. When these viruses have been invaginated and transported to somata and dendrites, they will be transformed to the other order of neurons without viral receptor, depending only on their neurotropic nature.
Another tract tracing technique is retrograde trans-synaptic tract tracing by inactivated pseudorabies virus (PRV) as tracers. The PRV is a neurotropic virus so they can enter a neuron, duplicate themselves, and spread from soma to terminals presynaptic to this soma. Intracellular tract tracing combined with electrophysiological studies is a large section of tract tracing methodology. Extracellular recording and tracer injection has also resulted in decent labeling following neuro-physiological identification.
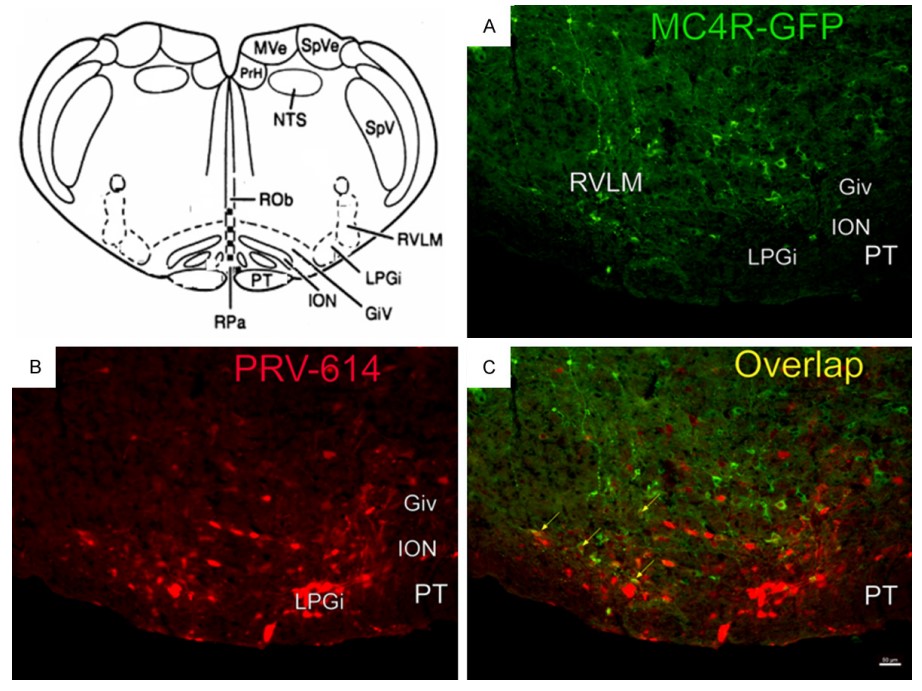
Fig.2 Sympathetic and melanocortinergic neurons in rostroventrolateral reticular nucleus (RVLM).
Creative Bioarray developed a series of novel neurotropic virus which can infect neuronal cells, duplicate and transmit along neuronal tract pathways. Neurotropic virus has many advantages over with traditional neuronal tract tracing tools:
Trans-synaptic properties
Controllable directions: both anterograde and retrograde
Almost no signal reduction after long distance tracing
Multiple indicators for choice.
These can be classified into two categories:
rhabdoviruses: rabies virus (RV) and vesicular stomatitis virus (VSV)
α-herpes virus: HSV-1-129 and pseudorabies virus (PRV)
for markers, we have EGFP, dsRed, mCherry, mRFP, tdTomato inserted in the RV, VSV, PRV backbones.
References
Yue CJ, et al. RVLM-IML pathway may implicate controlling peripheral airways by melanocortinergic-sympathetic signaling: A transneuronal labeling study using pseudorabies virus. Int J Clin Exp Pathol. 2014; 7: 7962–7966.
Huh Y, et al. Gene transfer in the nervous system and implications for transsynaptic neuronal tracing. Expert Opin Biol Ther. 2010; 10: 763–772.
Related Section
- Optogenetics
- Manual Patch-clamp Technique
- Automated Patch-clamp
- Multi-Electrode Array (MEA)
- FluxOR™ Thallium Assay
- FLIPR Detection System
Inquiry

