- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Acute/Cultured Organotypic Brain Slices
Besides cell cultures, brain slices are the most commonly used in vitro preparations for analysis of brain functions in mammals. Acute/Cultured organotypic slices, which are constituted by a quasi-monolayer of cells, retain their characteristic dendritic, axonal and synaptic morphology, as well as their connectivity and physiological and pathophysiological phenotypes.
Hippocampal slicing is the most extensively used brain tissue preparation in the study of the fundamentals of neurophysiology at cellular and synaptic levels due to simple and easy dissecting and cutting procedures and its well-known fiber connections and cytostructural. This preparation can be used to study neuronal biophysical and electrical properties, synaptic transmission, and plasticity in health and diseases. The hippocampal slice preparation is also used as a system to study brain metabolism, pharmacology of drugs in the CNS, and as a model for numerous pathological conditions.
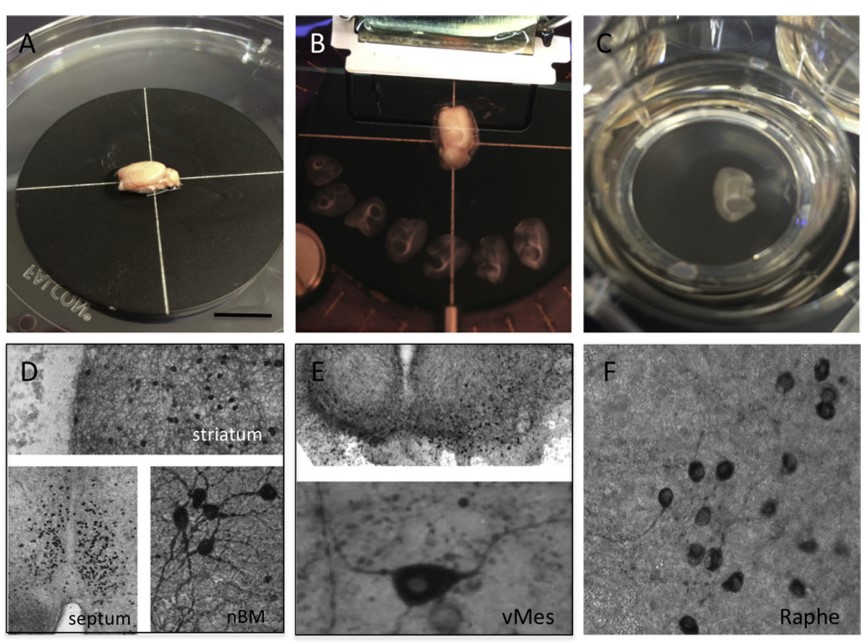
Fig. 1 Organotypic brain slices are prepared from whole postnatal or adult brains
In vitro brain slice preparation, taking the hippocampal slice as an example, offers certain advantages when compared to in vivo study including, but are not limited to:
Direct visualization of the tissue structure and visual control of the placement of stimulation and recording electrodes which would not be possible in an in vivo system
Maintenance of structure integrity needed for studying synaptic transmission and plasticity
Mechanical stability for whole-cell patch recordings or sharp electrode intracellular recordings due to lack of interference of heartbeat pulses and respiration
Easy manipulation and control of extracellular environment such as ionic composition, pH, osmolality, and temperature
No blood–brain barrier, which allows a direct action of drugs, neurotransmitters, and other compounds on neural cells when applied via bath perfusion
Maintaining structural connections that are lost in cell cultures and representing a simplified model of the neural circuit in situ
Easy extraction from rodent brains
Survive for hours in oxygenated artificial cerebrospinal fluid (ACSF)
All of these advantages make rodent hippocampal slices popular in the investigations of neurobiology, especially in the studies of synaptic transmission and plasticity. Both acute and cultured organotypic brain slices can be obtained in Creative Bioarray. In the case of organotypic brain slice cultures, these slices need to be cultured for at least 10-14 days to guarantee that they are not activated by endogenous release of calcium or glutamate and that reactive astrogliosis is minimized. Further, developing slices need time for maturation and stabilization of intrinsic axonal projections. Only such "resting non-activated" brain slices are useful for further investigation.
Multiple applications of Acute/Cultured organotypic brain slices in Creative Bioarray
Repeated multi-electrophysiological recordings and stimulations or gene transfer techniques
Retro-grade tracing using fluorescent dyes or long-term live imaging
Slices can be detached from the membranes and extracted (e.g. by sonification or lysis) for use for ELISAs, RT-PCR, HPLC etc. PAF-fixed slices are easy to handle and free- floating
Slices can be analyzed by in situ hybridization
Neuroprotection and neurotoxicity assays - can be easily used to test neuroprotective molecules such as e.g. growth factors or neuroactive drugs
Models for neuro-toxicological screenings.
Among all the applications of brain slices, electrophysiological recordings are most frequently used as a direct way to probe neural function, detect pathological functional abnormalities and explore sensitivity of spontaneous and evoked electrical signal transmissions to drugs. Electrophysiological recordings from brain slices are a direct way to probe neural function, detect pathological functional abnormalities and explore sensitivity of spontaneous and evoked electrical signal transmissions to drugs.
The recordings are a valuable tool in the study of synaptic plasticity, which is thought to underlie learning, memory and some brain pathologies. Up- or down-regulation of synaptic transmission can shed light on cellular and network mechanisms of numerous CNS diseases, including Alzheimer's disease, autism and ataxia. Slice electrophysiology can also be used to identify specific drug targets, evaluate efficacy, investigate mechanism of action and thus aid assay development to translate such findings into high and medium throughput screens.
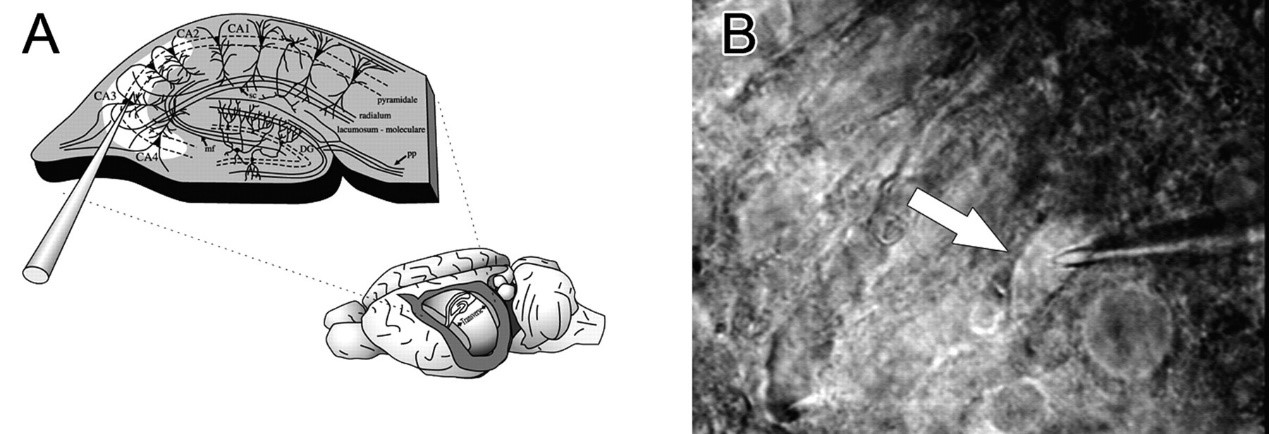
Fig.2 Hippocampal slice preparation and single cell cytoplasmic extraction
Assays
Slice electrophysiology at Creative Bioarray includes:
Blind or infrared visualized patch clamp recordings from brain and spinal cord slices
Analysis of drug action on excitatory and inhibitory pre- and post-synaptic activity, both fast (ligand gated ion channel-mediated) and slow (e.g. GPCR-mediated)
Direct measurement of drug action on cell excitability
Extracellular recordings, including measurement of long-term potentiation and long-term depression in, for example, hippocampal slices using field potential recordings
Multi-electrode array (MEA) recording 128 channels simultaneously
Highlights
A broad range of pharmacological and physiological data will be provided by Creative Bioarray's slice experts, using the following techniques:
Current/voltage-clamp recordings
Perforated patch recordings
Pre-/post-synaptic activity
Paired recordings
Field potential recordings
Quantitative/single-cell RT-PCR
References
Jurgens CWD, et al. Alpha2A adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region. Mol Pharmacol. 2007; 71: 1572–1581.
Humpel C. Organotypic brain slice cultures: A review. Neuroscience. 2015; 305: 86–98.
Related Section
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
Inquiry

