- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Cardiac in vivo Assays
In cardiac safety studies, hERG testing is positioned early such that lead structures are evaluated prior to entering lead optimization for hERG-blocking potential. Lead optimization programs are supported with additional studies to examine the myocardial action potential and manual patch-clamping studies to determine IC50 values for hERG blockade for interesting candidates.
In vivo studies are done toward the end of the lead optimization process on selected candidates for final characterization and selection. GLP studies are done only for development compounds being prepared for clinical trials. Creative Bioarray has conducted in vivo testing for cardiovascular disease using in vivo models for both side-effect profiling and efficacy according to your requirement.
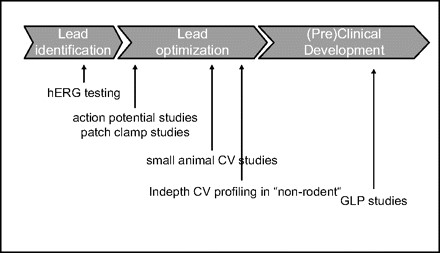
Fig. 1 Depiction of the timing of various safety pharmacology studies for cardiovascular liabilities
Creative Bioarray provides a variety of study endpoints to generate the high-quality, reproducible data according to your research program requirement. All test models are analyzed in a dose-dependent manner with approved benchmark positive controls. Each of our technical directors specializes in selected research areas so they will provide expert scientific support to you. They are involved in all process including consulting with you to identify an appropriate model to meet scientific objectives, designing studies and writing protocols and statements of work (SOWs) to meet the quality assurance guidelines. They also work closely with our study directors to assure that SOWs are clear and that all methods and timelines are feasible. To meet the needs of your specific Cardiovascular drug discovery project we also have the flexibility and expertise to develop custom in vivo models.
We provide protocols including screening study designs for preliminary safety assessment, ECG/MAP/LVP, Coronary flow/pressure, MAP duration, QT & QRS duration, LVDP, MAP triangulation (instability), frequency-dependence & restitution, effective refractory period. Whether you are trying to evaluate potential cardiovascular liabilities during lead optimization or trying to verify in vivo efficacy, we can provide high quality and reproducible results to progress your drug discovery program.
Highlights
Both GLP compliant studies for IND submission and non GLP compliant studies available
Multiple Species
Telemetry (Invasive and Non-Invasive; ECG and systemic/ventricular pressures)
Cardiac Output and Chronic Load-Independent Cardiac Function
Imaging (Echocardiography, CT, MRI, x-ray)
Applications
QRS,QT waves Detection by Electrocardiogram
Heart Rate (HR, bpm)
PR interval (ms)
QRS interval (ms)
QT and corrected QT
Cardiac Activity Evaluation
Ejection fraction
Heart Structure
Basic Cardiovascular Functions
Arterial Blood Pressure (ABP, mmHg)
Left Ventricular Pressure (mmHg)
Customized Services
Quotation and Ordering
If you have any special needs in our Cardiac in vivo Assay Service, please contact us at Email or Telephone for this special service. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
Reference
Guth, B. D. Preclinical cardiovascular risk assessment in modern drug development. Toxicol Sci. 2007; 97: 4–20.
Related Section
Inquiry

