- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
CiPA
In evaluation of cardiac toxicity of drug candidates, many drugs with effects on hERG and QTc prolongation but little actual Torsades de pointes (TdP) risk are deprioritized or excluded from further development or, if approved, see their clinical use limited by inappropriate warnings in product labeling.
Here at Creative Bioarray, we provide our clients with a comprehensive pack of CiPA evaluation service.
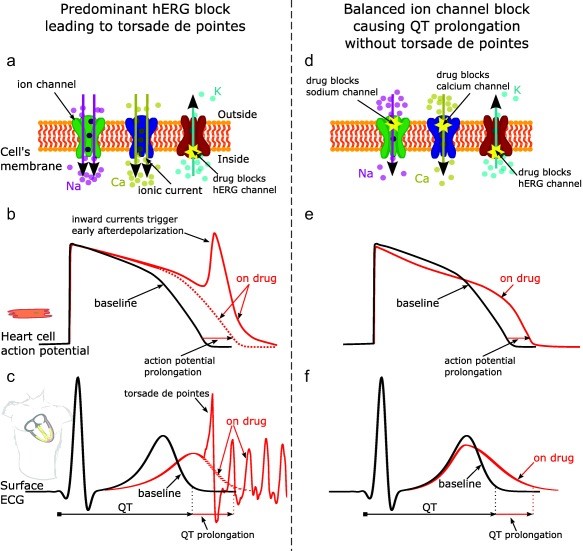
Fig. 1 Effect of predominant hERG potassium channel block vs. balanced ion channel block on QT prolongation and generation of torsade de pointes
The duration of ventricular repolarization is determined by a fine balance between inward (depolarizing) and outward (repolarization currents) that are activated with each heartbeat. Drugs that affect both inward and outward cardiac currents (e.g. verapamil, ranolazine) may therefore have effects on repolarization and the QTc interval, but not be associated with the generation of TdP due to the blockade of inward currents.
The Comprehensive In Vitro Pro-Arrhythmia Assay (CiPA) represents a paradigm shift towards a more complete assessment of proarrhythmic risk rather than QT prolongation alone. CiPA is a novel cardiac safety screening proposal that evaluates drug effects on each cardiac ion channel type individually (high-throughput), predicts net effect on the cardiomyocyte action potential with computer modeling, and then verifies the in silico conclusions using human stem cell-derived cardiomyocytes.
Work Flow
Automated electrophysiology screening against an expanded panel of six cardiac ion channels, including hERG.
In silico modelling using electrophysiology data from the expanded ion channel panel.
Confirmation of in silico predictions using translational assays employing human iPSC-derived cardiomyocytes.
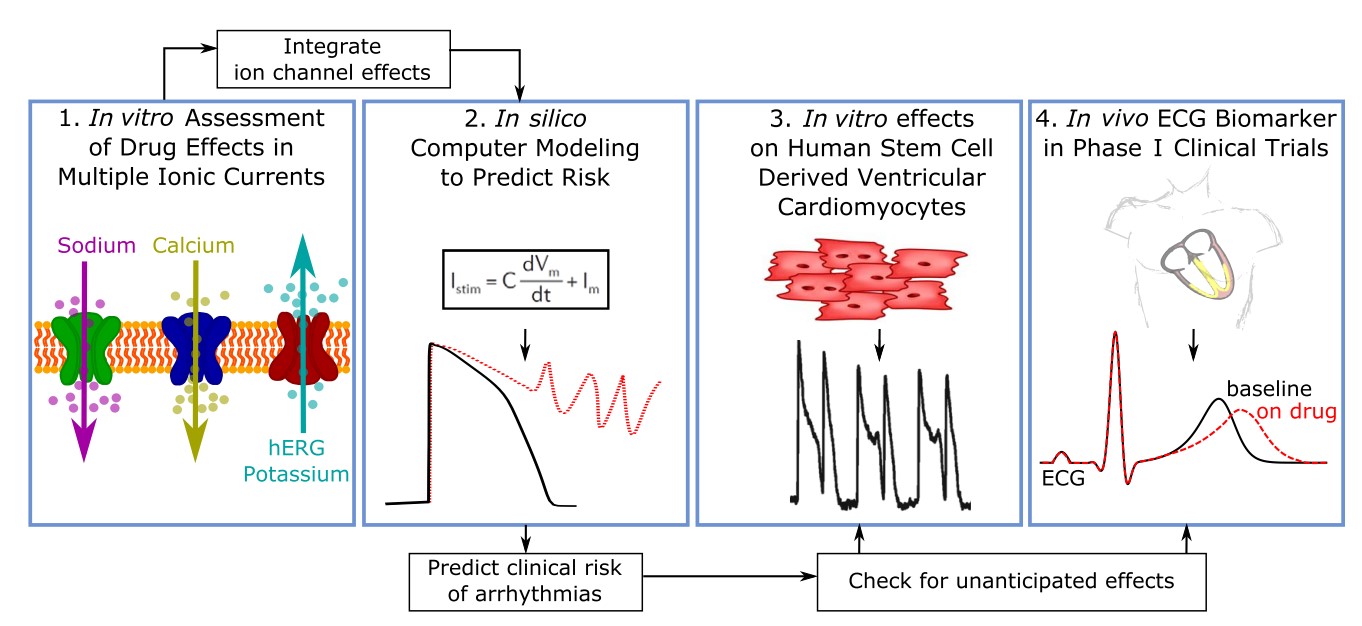
Fig. 2 The CiPA Workflow
Quotation and Ordering
If you have any special needs in our CiPA Service, please contact us at Email or Telephone for this special service. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
References
Vicente J, et al. Mechanistic Model-Informed Proarrhythmic Risk Assessment of Drugs: Review of the ‘CiPA' Initiative and Design of a Prospective Clinical Validation Study. Clin Pharmacol Ther. 2018; 103: 54–66.
Sager PT., et al. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 2014; 167: 292–300.
Related Section
Inquiry

