- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
iPSC-derived Cardiomyocytes
For most arrhythmia syndromes, clinical management is hindered by insufficient knowledge of the functional consequences of the mutations. Disease expressivity and sensitivity to therapeutic interventions often varies between mutations and/or patients, underlining the need for more individualized strategies.
Cardiomyocytes (also known as myocardiocytes or cardiac myocytes) are the muscle cells (myocytes) that make up the cardiac muscle. Due to the lack of natural source of cardiac cells, in vitro differentiated human cardiomyocytes have been proven as an essential tool not only for general cardiovascular research, but also as drug development and pre-clinical research.
Creative Bioarray provides ready-to-use beating human induced pluripotent stem cells (iPSC)-derived cardiomyocytes. Generated from mature cells that have been genetically reprogramed to a pluripotent stem cell state, induced pluripotent stem cells (iPSCs) can be readily expanded and induced to specialize or differentiate into cardiomyocytes in vitro.
The development of the induced pluripotent stem cell (iPSC) technology now provides the opportunity for generating iPSC-derived cardiomyocytes (CMs) from human material (hiPSC-CMs), enabling patient- and/or mutation-specific investigations. These hiPSC-CMs may furthermore be employed for identification and assessment of novel therapeutic strategies for arrhythmia syndromes. Patient-derived iPSCs offer an advantage over traditional in vivo cardiac disease modeling as the reprogrammed cells incorporate the complex genetics associated with cardiac disease. In an iPSC patient-specific disease model, patient cells are harvested and genetically reprogrammed to produce disease-specific iPSC lines. iPSC-CMs are considered ideal for disease modeling and clinical applications as they possess a robust proliferation capacity that is ideal for scalability and may be readily employed where other pluripotent cells like embryonic stem cells are prohibited.
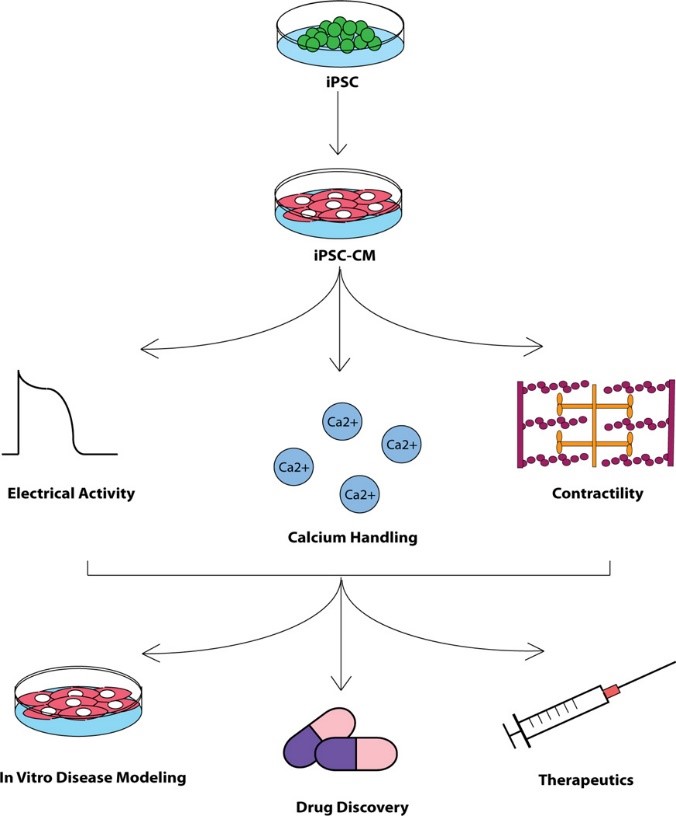
Fig. 1 Overview of experimental flow utilizing hiPSC-CMs
These hiPSC-CMs in Creative Bioarray enable investigation of patient- and/or mutation-specific disease mechanisms as well as identification and assessment of novel therapeutic strategies for arrhythmia syndromes. Creative Bioarray also provides customized differentiation service.
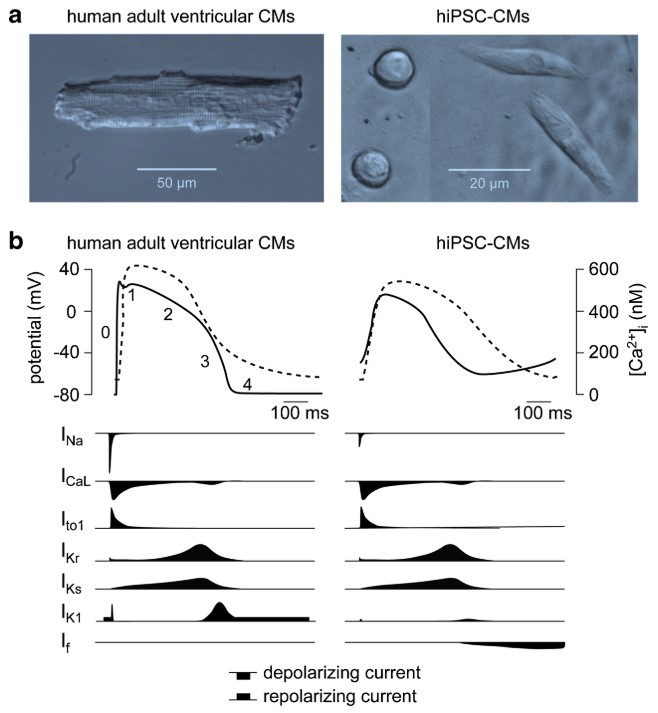
Fig. 2 Morphological and electrophysiological phenotype of human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and native human ventricular cardiomyocytes (CMs)
Applications
Independent assays: From electrophysiology and multi-electrode array analysis (MEA) to high content microscopy and viability screens.
Cardiac Disease modeling: To date, interesting phenotypes of several genetic-based cardiomyopathies have been successfully replicated in a dish through the use of patient-specific iPSCs. Moreover, the novel safety screening proposal led by FDA, Comprehensive in vitro Proarrhythmie Assay (CiPA) intended to improve current regulatory guidance. The new predictive technologies are evaluated, including using human stem cell-derived cardiomyocytes (hSC-CMs) in the preclinical cardiac safety assessment.
Cardiac safety and toxicity: Cardiac toxicity is an important factor for the failure of therapeutic agents in late stages of clinical trials, as well as for the removal of approved drugs from the market. Through the CiPA initiative, which brings together researchers from institutions such as the FDA, academic institutions and pharmaceutical companies, hiPSC-CMs are being evaluated as an integral tool for the safety assessment of developing drugs.
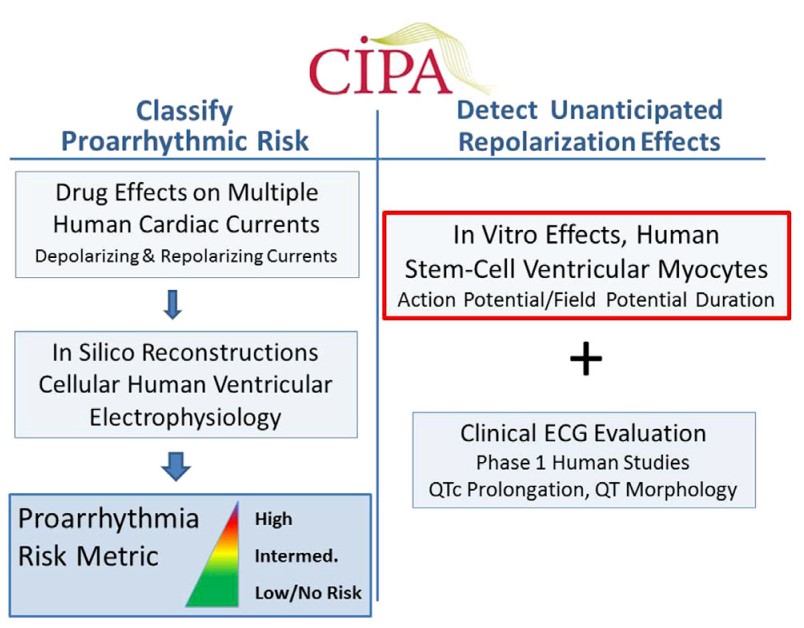
Fig. 3 The role of human stem cell-derived cardiomyocytes in the CiPA paradigm
Characterization
Post-thawing viability and plating efficiencies of the cells are typically higher than 50%. These iPSC-Derived Cardiac Cells are also tested to ensure the absence of microorganism contaminants.
Purity: higher than 90%.
After plating at the recommended density, the cells show spontaneous synchronized beating.
The cardiomyocyte content of our preparations is determined by flow cytometry analysis and quantification of cardiac troponin T and sarcomeric alpha actinin (SaAct) positive cells.
Functional validation: by patch-clamp and Fluo-4 Direct Calcium Assay.
Begin beating within 3 days after thawing and assay-ready within 10 days post-replating.
QC validation: negative for mycoplasma, bacteria, yeast, and fungi. HIV-1, hepatitis B and hepatitis C.
Each cell lot with specific basic donor information (gender/age/race).
Tests
Electrophysiological Measurements: evaluate the electrical impulses that become irregular in diseased cardiomyocytes. MEA measures voltage across a population of cardiomyocytes to determine duration QT intervals. The Patch clamp approach, on the other hand, monitors individual ion currents (e.g., INa, ICa) and may be useful in evaluations of ion channel function.
Contractile Evaluation: One of the notable in vitro iPSC-CMs hallmarks is the active contraction of cardiomyocytes in the dish. Video microscopy and motion tracking software are used to capitalize on this feature and quantify the beating frequency, amplitude, and kinetics of contractile cardiomyocyte clusters.
Calcium dependent Assays: Cyclical Ca2+ released from and reuptake by the sarcoplasmic reticulum drives the excitation-contraction coupling process are responsible for cardiomyocyte contraction. As such, calcium-sensitive Fluo-4 and Fura-2 dyes may be used to detect the initial Ca2+ release events that ultimately produce contractile beating clusters.
References
Casini S, et al. Human iPSC-Derived Cardiomyocytes for Investigation of Disease Mechanisms and Therapeutic Strategies in Inherited Arrhythmia Syndromes: Strengths and Limitations. Cardiovasc Drugs Ther. 2017; 31: 325–344.
Veerman CC, et al. Immaturity ofhuman stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem? Stem Cells Dev. 2015; 24:1035–52.
Bedada FB, et al. Maturation status of sarcomere structure and function in human iPSC-derived cardiac myocytes. Biochim Biophys Acta - Mol Cell Res. 2016; 1863: 1829–1838.
Gintant G, et al. The Evolving Roles of Human iPSC-Derived Cardiomyocytes in Drug Safety and Discovery. Cell Stem Cell. 2017; 21: 14–17.
Related Section
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
Inquiry

