- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Achromatopsia
Achromatopsia, also known as rod monochromacy and total congenital color blindness. It is a medical syndrome that usually exhibits at least five related symptoms, such as color blindness, amblyopia, hemeralopia, nystagmus, and abnormal iris operation. The term originally referred to acquired diseases, such as disorders related to damage to the midbrain (mainly the midthalamus or cerebral cortex, especially the fourth visually related area, V4), but now it usually refers to autosomal recessive inherited congenital visual diseases, where patients cannot perceive color or achieve satisfactory vision under external sunlight. Congenital patients with this condition showed no cone activity at all at high light levels through electroretinography. Existing studies have found that there are at least four genetic mutation causes of congenital color blindness, two of which involve cyclic nucleotide-gated ion channels (ACHM2, ACHM3), and the third involves cone photoreceptor transduction proteins (GNAT2 and ACHM4) , the last is unknown.
Causes of Achromatopsia
Through molecular biology research, it was found that congenital color blindness is caused by mutations in various genes involved in cone cell function. These genes include cyclic nucleotide-gated channel beta 3 (CNGB3 / ACHM3), Cyclic nucleotide-gated channel alpha 3 (CNGA3 / ACHM2), guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 2 (GNAT2 / ACHM4), phosphodiesterase 6C (PDE6C / ACHM5), and phosphodiesterase 6H (PDE6H / ACHM6). After the photons are absorbed by opsin, the 11-cis retinal in opsin is converted to all-trans retinal. This process activates opsin, which in turn activates the transduction protein GNAT2 (ACHM4) consisting of three subunits (α, β, and γ). The activated transduction protein in turn activates phosphodiesterase 6 (PDE), which is composed of the inhibition of α and β subunits (or two α subunits) and two γ subunits. The activated transduced protein binds to its γ subunit to release the active PDE (6C or 6H). The activated PDE hydrolyzes cGMP to GMP. cGMP keeps cyclic nucleotide ion channels (CNG, tetramers with alpha and beta subunits) open. Eventually, the polarization of the membrane changes, so that the color signal can be transmitted to the brain.
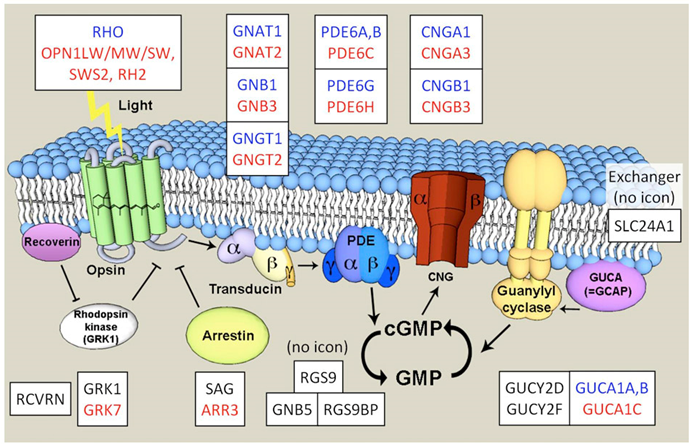 Figure 1. Schematic outline of the phototransduction cascade in a vertebrate cone(Larhammar, D., K.; et al. 2009).
Figure 1. Schematic outline of the phototransduction cascade in a vertebrate cone(Larhammar, D., K.; et al. 2009).
Pathophysiology of Achromatopsia
In general, the molecular pathogenesis of color blindness cannot correctly control or respond to changes in cGMP levels. The study found that the level of cGMP is particularly important visually, because its level controls the opening of cyclic nucleotide-gated ion channels (CNG). Decreasing the concentration of cGMP will cause the closure of CNGs, resulting in the cessation of hyperpolarization and glutamate release. Natural retina CNG is composed of 2 α-subunits and 2 β-subunits in cone cells, which are CNGA3 and CNGB3, respectively. The study found that the channels produced by the combined assembly of CNGA3 and CNGB3 have altered membrane expression, ion permeability (Na+ and K+ and Ca2+), cAMP / cGMP activation, reduced external image correction, current flicker, and L-cis Blockade and sensitivity to diltiazem. Mutations in patients with color blindness often lead to the loss of CNGB3 function or the acquisition of function, often increasing the affinity of CNGA3 for cGMP, causing the loss of function of the gating effect. In addition, cGMP levels are controlled by the activity of cone cell transduction protein GNAT2. GNAT2 mutations often result in a truncated, possibly non-functional protein, which hinders the effect of photons on cGMP levels, thereby blocking the transmission of color signals. Interestingly, the study found a positive correlation between the severity of these protein mutations and the integrity of the color blind phenotype.
Recent Research Status
With the establishment of adeno-associated virus (AAV) -based treatment of mouse color blindness models (CNGB3, CNGA3 or GNAT2 gene mutations), several cone-specific promoters have recently been developed and have been used in mice and non-human primates Success. In addition, the sheep CNGA3 model was also characterized. Two clinical trials are currently underway: one is to better characterize human color blindness, and the other is to study a ciliary neurotrophic factor (CNTF) implant for the treatment of patients with CNGB3 mutations.
References
- Larhammar, D., K.; et al., Evolution of vertebrate rod and cone phototransduction genes. Philos Trans R Soc Lond B Biol Sci. 2009, 364: p. 2867-80.
- Bright, S. R.; et al. Disease-associated mutations in CNGB3 produce gain of function alterations in cone cyclic nucleotide-gated channels. Mol. Vis. 2005,11: 1141–1150.
- Patel, K. A.; et al. Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel. Investig. Ophthalmol. Vis. Sci. 2005, 46 (7): 2282–2290.
Related Section
Inquiry

