- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Acute Isolated Cardiomyocytes Technique
The use of adult cardiomyocytes isolated from rat and other animal cardiac tissue has been the gold standard for in vitro studies of cardiac electrophysiology since the 1970s. It is well known that the patch clamp technique can be used to record a variety of ionic currents on cardiomyocytes, such as potassium current, sodium current, calcium current, and sodium-calcium exchange current. The key to its success depends on the formation of a gigaseal between the cell membrane and the glass microelectrode. This largely depends on the quality of the cells. Existing studies have shown that many factors such as water, enzymes, temperature, pH, etc. can affect the quantity and quality of isolated cells.
The Workflow of Acute Isolated Cardiomyocyte Technique
When separating cells, first perfuse with calcium-free solution for 7-8 minutes to decalcify the myocardial tissue, make the tissue loose, and then digest it with enzymes. The resulting single cells were preserved in a high potassium KB solution to reduce the energy metabolism of the cells. Such cells need to be perfused and recalcified with normal Tyrode's solution before sealing, so that the cardiomyocytes can be restored to normal state before recording various currents and action potentials of cardiomyocytes. During the recalcification process, some cells will continue to contract, and some cells will die. Only those cardiomyocytes that do not contract, have clear striated, and long rods can be used for patch-clamp experiments. Therefore, the isolated single cardiomyocytes must have both a certain quantity and a certain quality in order to ensure the smooth progress of the patch clamp experiment.
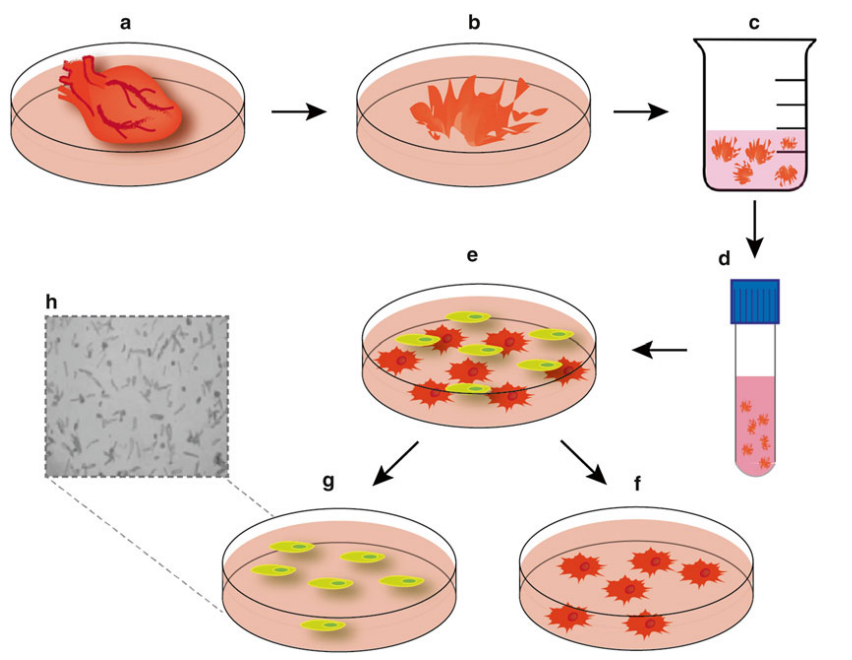
Figure 1. Schematic for isolation of neonatal cardiomyocytes and cardiofi broblasts. (Puthalakath, Hamsa; et al.; 2016)
Influencing Factors of Acute Cardiomyocyte Isolation Technique
Water Quality Requirements for Isolated Cardiomyocytes
Water is a decisive factor in the success rate of isolated cells. If the water quality is not good, and the conductance is too high, the cells cannot be separated. When performing cardiomyocyte isolation, distilled or deionized water should be used. The conductance of water is required to be below 1 μS/cm. The use of qualified deionized water can greatly promote the successful isolation of cardiomyocytes.
Enzymes for Isolating Cardiomyocytes
Enzymes are also a key factor in the success rate of isolating cells. The potency and amount of enzyme greatly affects the quantity and quality of isolated cells. Too much collagenase can damage the structure of the cells themselves. As a result, the obtained cells were enlarged in volume, the cell structure was blurred, and the horizontal stripes disappeared. During the recalcification process, a large number of cells will die, and a small number of surviving cells will rupture the membrane before the high-resistance sealing is reached, and patch clamp experiments cannot be performed. Too little enzyme was added, and the junctions between cells were not digested. During pipetting, the small pieces of myocardial tissue cut out were in the form of flocs that were not easily dispersed. With vigorous pipetting, there will be more cell debris and dead cells, but almost no living cells with clear long rod-shaped structures. The appropriate amount of dosage needs to be constantly explored in practice.
Temperature Conditions for Isolating Cardiomyocytes
Temperature also plays an important role in the success of isolating cells. Immediately place the heart in ice-cold calcium-free Tyrode's solution when removing the heart. This can weaken the metabolism of myocardial tissue during pruning, thereby protecting myocardial tissue; during the separation process, the room temperature should not be lower than 20 °C, and the temperature of the perfusion device should be between 38 and 39 °C. This keeps the temperature of the fluid flowing out of the heart around 34°C. If the temperature is too high (>35°C), the separated cells will loose the structure, expand the volume and lose the three-dimensional structure. In this process, although there are individual living cells that can be subjected to patch clamp experiments, after sealing and membrane rupture, the cells cannot last for a long time, and they shrink into a ball and die soon. If the temperature is too low (<32°C), although the cell survival rate is high, even up to 100%, some undigested substances remain on the membrane. This makes the cell membrane not smooth enough, affecting the sealing with the glass microelectrode, or almost impossible to seal, resulting in difficult membrane rupture.
Other Influencing Factors
The speed of picking the heart should be fast, preferably within 3 to 4 minutes; air bubbles must be expelled in the perfusion tube, if the air bubbles block the coronary artery during the perfusion process, the myocardial tissue in this part will be infarcted. The color of the tissue will turn black, and the cells will be hypoxic; although the structure of the hypoxic cells looks good, they cannot be sealed, and the cells will shrink and die when the electrodes are attached. The pH value and O2 filling of the perfusate are also important factors; if the perfusate is too acidic, too alkaline or hypoxia, cells suitable for patch-clamp experiments cannot be obtained. In short, when isolating cells, every step must be carefully and carefully, any mistakes will affect the success rate of isolating cells, which will directly affect the patch clamp experiment.
References
- Sakai M, et al.; Contractile response of individual cardiac myocytes to norepinephrine declines with senescence. Am J Physiol. 1992, 262: H184.
- Louch WE, et al.; Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011, 51(3):288-298.
- Puthalakath, Hamsa; et al.; [Methods in Molecular Biology] Programmed Cell Death Volume 1419 || Isolation of Cardiomyocytes and Cardiofibroblasts for Ex Vivo Analysis. ,10.1007/978-1-4939-3581-9(Chapter 10), 2016, 117–129.
Related Section
Inquiry

