- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Application of Xenopus Oocyte Model in the Study of Ion Channels
Using tractable organisms to answer fundamental questions in medicine and biology has been a common practice since ancient times. One of the model systems with important contributions is the African clawed frog, a pseudotetraploid vertebrate living in freshwater. The reason for its worldwide use for research is its high conservation of the most important cellular and molecular mechanisms. Furthermore, it is inexpensive, easy to handle, and readily obtains a large amount of material for various experimental procedures.
Xenopus Oocytes
Xenopus reproductive process can be manipulated so that each Xenopus can lay 3-4 eggs per year, and the material used in the study can range from oocytes to free-cell extracts. The eggs of Xenopus laevis were produced by subcutaneous injection of 50-100 units of pregnant mare serum (PMS) into their dorsal lymph sac. An injection of 600-800 units of human chorionic gonadotropin (hCG) is performed 12-16 hours before ideal spawning. After the second injection, they were placed in separate containers, and the eggs were collected the next day.
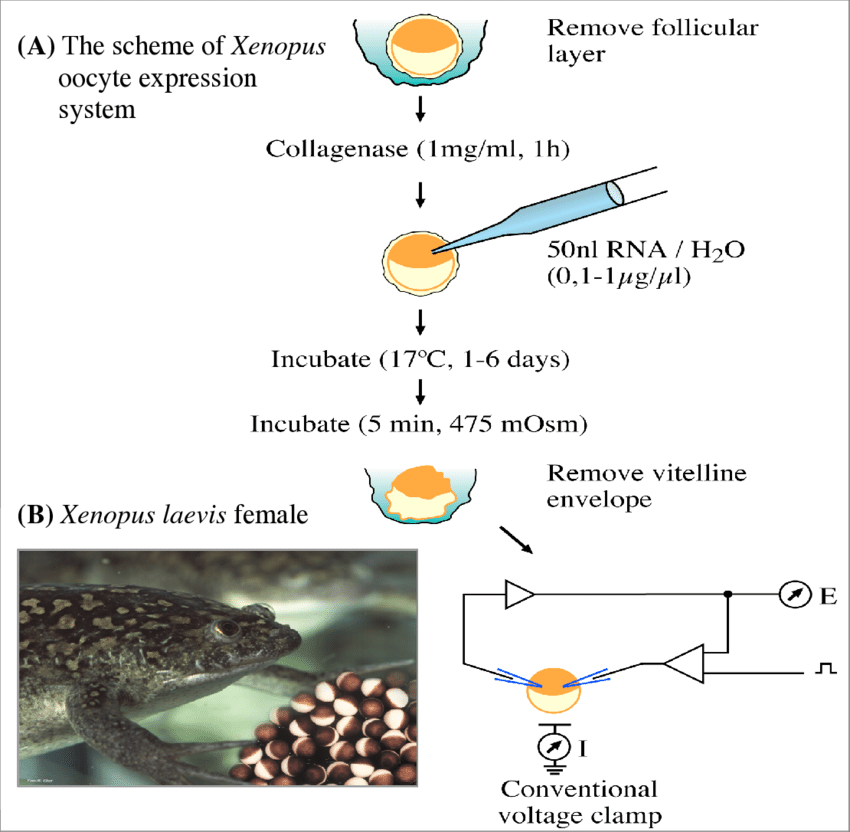
Figure 1. The artificial expression of ion channels on the membrane of Xenopus laevis oocytes. (Ahmed Al-Sabi, et al.;2004)
The large, transparent ovaries of Xenopus laevis, which may contain hundreds of oocytes of the same size, are easily obtained. Xenopus oocytes are large cells greater than 1 mm in diameter. They contain a giant nucleus (or vesicle) that is 100,000 times larger than the nucleus of a somatic cell, accounting for about one-third of the oocyte's volume, and develop rapidly. The vesicles are surrounded by a nuclear membrane, which has large pores to facilitate substance transport in and out of the cytoplasm. Despite differences in size, the processes of oogenesis and maturation are conserved among mammals. Therefore, they are widely used by researchers studying the cell cycle. Isolation and enucleation of oocytes Oocytes can be either manually isolated from the ovary with forceps or extracted by collagenase digestion of the surrounding four layers of extracellular connective tissue. This yields oocytes without nuclei and isolated nuclei. The isolated fully functional nuclei can be used to study all nuclear processes, including gene expression, chromatin dynamics, nuclear import and export of fluorescently tagged proteins, or the function of nuclear pore complexes.
Biological Applications of the Xenopus Oocyte Model
Two-electrode Voltage Clamp Technique in Xenopus Oocytes
Ion channels in the cell membrane can be gated by membrane potential (Vm) and/or by specific chemicals. Those channels belonging to the voltage-dependent ion channel superfamily are directly gated by Vm, while others classified as ligand-gated ion channels are gated by their binding to specific ligands. On the other hand, voltage-dependent ion channels can be regulated by chemical factors such as neurotransmitters, hormones, intracellular messengers, or exogenous drugs; while some ligand-gated ion channels (such as NMDA receptors) are also affected by V m changes Impact. Therefore, in order to conveniently study the voltage-dependent properties of ion channels, or to differentiate the effects of Vm from chemicals, an experimental procedure is required to control Vm (ie, change Vm in a desired pattern or set it to a desired s level). This procedure is called voltage clamp.
The voltage clamp was originally designed by Cole and Marmont and improved by Hodgkin, Huxley and Katz for its application to giant axons in squid as two-electrode voltage clamp (TEVC) in the late 1940s. It utilizes two intracellular electrodes, one for monitoring V m and the other for injecting current to adjust V m to the desired value (the injected current is equal to the membrane current). While the use of TEVC is limited to giant axons or large cells such as skeletal muscle cells, much of scientists' understanding of the basic biophysical properties of ion channels has been gained by using this method. It is based on this method combined with experiments using extracellular electrodes that Neher and Sakmann developed the widely used single-electrode patch-clamp technique. Although the single-electrode patch clamp technique is more popular now, it is only suitable for clamping relatively small cells, not for large cells. Because the high current will cause a significant voltage drop across the recording electrode, it cannot be compensated to an acceptable level. Therefore, TEVC remains irreplaceable in voltage-clamping large cells, especially Xenopus oocytes commonly used for exogenous expression of ion channels or receptors. Xenopus oocytes are a convenient expression system that is widely used to study the structure and function of ion channels and receptors using TEVC in combination with various molecular biology approaches, as its membrane has low expression of endogenous channels and receptor.
Incisional Oocyte Voltage Clamp Technique (COVG)
Xenopus oocytes are widely used as an expression system for ion channels. Scientists have also developed the open oocyte voltage clamp technique (COVG), which has relatively low current noise (~1-nA rms at 5 kHz) and can charge membrane capacity in 20-40/xs, and make it possible to control the intracellular environment through the internal perfusion of the oocyte. Another feature is the ease of obtaining stable recordings that last for hours. These properties allow sufficient resolution of the time course of fast ions and gated charging currents.
References
- Stefani, Enrico (1998). [Methods in Enzymology] Ion Channels Part B Volume 293 || (17) Cut-open oocyte voltage-clamp technique. 300–318.
- Gamper, Nikita (2013). [Methods in Molecular Biology] Ion Channels Volume 998 || Two-Electrode Voltage Clamp. 10.1007/978-1-62703-351-0(Chapter 6), 79–89.
- Ahmed Al-Sabi, et al.; Structural and functional studies of kappa M-conotoxin RIIIK interaction with Shaker-related potassium channels from trout fish (TSha1). 2004, https://www.researchgate.net/publication/27335866.
Related Section
Inquiry

