- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Autoimmune Autonomic Ganglionopathy
Autoimmune autonomic ganglionopathy (AAG) is an immune-mediated acquired autonomic dysfunction disease that is related to auto-acetylcholine receptor antibodies and mainly manifests as autonomic dysfunction. The disease mostly occurs in young and middle-aged patients, and it often has prodromal symptoms such as respiratory or digestive tract infections, mainly manifested as impaired sympathetic nerve function, such as orthostatic hypotension, syncope, sweatlessness and other glandular secretory dysfunction; paraautonomic dysfunction, such as dry mouth, dry eyes, pupils with slow light reflection, bladder dysfunction, etc.; and gastrointestinal dysfunction, such as gastrointestinal motility dysfunction, constipation, gastroparesis, pseudointestinal obstruction, etc. The autonomic dysfunction caused by the disease is disabling and sometimes even threatens the life of the patient. According to a survey by the National Autonomic Dysfunction Research Foundation, there are currently more than 1 million patients with autonomic dysfunction, of which AAG is an important cause. The pathogenesis of AAG is not yet clear. Gibbons believes that most AAGs are closely related to ganglion nicotinic acetylcholine receptor (nAChR) antibodies, and that ganglion AChRs antibodies are present in nearly 50% of patients with idiopathic autonomic nerve disease.
Ganglion AChR antibody and AAG pathogenesis
In the process of synaptic conduction in autonomic ganglia, presynaptic neurons release acetylcholine for post-synaptic AChR to mediate rapid synaptic transmission. Nicotinic acetylcholine receptors are receptor polypeptides that respond to the neurotransmitter acetylcholine. They exist in the central and peripheral nervous systems, muscles, and many other tissues of many organisms. At the neuromuscular junction they are the main receptors in the muscle for communication between motor nerves that control nerve contraction and muscles. AChRs are members of the ligand-gated cation channel family, and are distributed throughout the central and peripheral nervous systems. AChRs usually consist of two α3 subunits combined with β2, β4 or α5 subunits. And mediate rapid synaptic transmission in the peripheral autonomic ganglia where AChR is located.
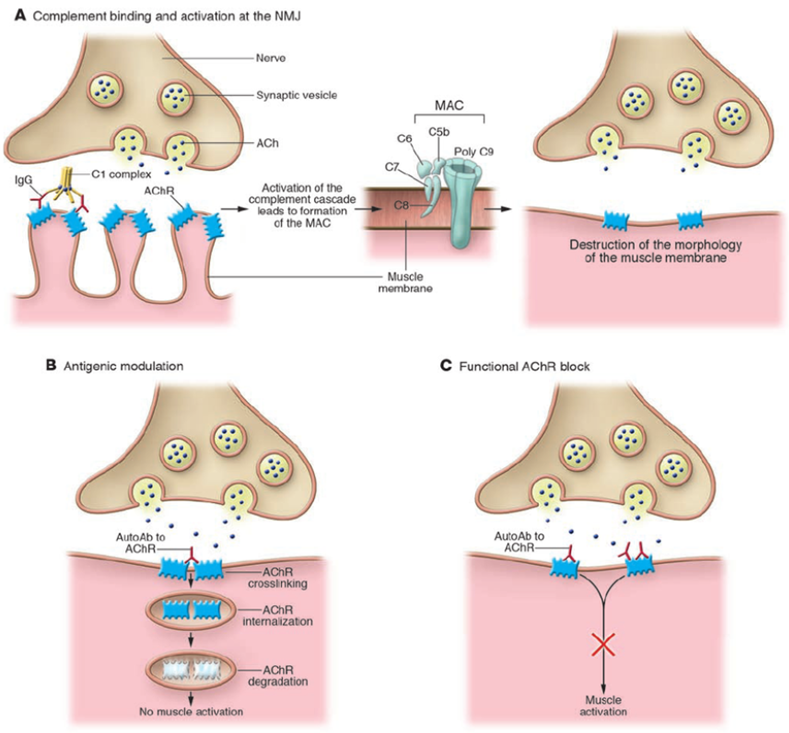
Figure 1. Effector mechanisms of anti-AChR Abs. (Bianca M. et al. 2006)
The clinical study of AAG disease has found that the degree of autonomic neuropathy is proportional to the plasma nAChR antibody concentration in patients with AAG; immunization of experimental rabbits with nAChRα3 subunit protein can cause autonomic dysfunction, and its symptoms are similar to AAG; the experimental mice are passively input with nAChRIgG antibody It can cause autonomic dysfunction; after exposure of neuroblastoma cells to IgG antibody in patients with autoimmune autonomic neuropathy, the AChR current in the post-synaptic membrane gradually decreases. An experimental AAG model has been established in animals, and rabbits or mice infused with ganglion AChR-IgG by active infusion of AChRα3 subprotein will have symptoms of autonomic neurological dysfunction similar to those of AAG patients. These studies fully prove that AAG is an autoimmune autonomic dysfunction disease mediated by nAChR antibody. In addition, two weeks after the passive inoculation of the AChR antibody, a similar situation was seen in the isolated sympathetic ganglia of mice: the post-synaptic neuronal response in mice was significantly weakened. It shows that these antibodies can directly cross-link with the post-synaptic ganglion AChR without internal complement and cause pathogenic.
References
- Lu B, et al.; α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular Medicine. 2014, 20 (1): 350-8.
- Steven Vernino, et al.; Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Autonomic Neuroscience. 2009, 146 (1-2): 3-7.
- Purves D, et al.; Neuroscience (4th ed.). Sinauer Associates. 2008, pp. 122-6.
- Bianca M. et al.; Myasthenia gravis: past, present, and future. J. Clin. Invest. 2006, 116:2843-2854.
Related Section
Inquiry

