- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Episodic Ataxia
Episodic ataxia (EA) is a rare type of autosomal dominant genetic disease, mainly manifested as self-limited cerebellar dysfunction with almost no fixed or progressive neurological abnormalities. Since Parker first reported 11 cases of patients with paroxysmal ataxia in 1946, many scholars have discovered similar families and described them. In recent years, family gene linkage analysis and electrophysiological studies have found that such diseases are caused by mutations in genes encoding ion channels. It is divided into the following categories according to gene location and accompanying symptoms: 1, paroxysmal ataxia type 1 (EA1), paroxysmal ataxia with muscle fibrillation (EA/myokymia), its gene is located at 12p13, which encodes potassium Gene mutations in ion channels. 2, Paroxysmal ataxia type 2 (EA2), paroxysmal ataxia with nystagmus (EA2/nystagmus) is caused by mutations in the gene encoding calcium channels at 19p13. 3, Paroxysmal choreoathetosis with episodie ataxia, the gene is located on 1p, and is related to potassium channels.
EA1
Clinical Manifestations
The main characteristics of this disease are transient ataxia, dysarthria, and clonus of the distal limbs lasting several seconds to several minutes. It can be accompanied by partial epilepsy. Fright and exercise can induce and aggravate seizures. Symptom occurs 1 to several times a day, up to 15 times. There are twitches or fibrillation of facial muscles and distal limb muscles during the interictal period. The electromyogram showed continuous spontaneous repetitive discharge. It usually starts in childhood or adolescence and gradually decreases with age. It is not accompanied by progressive deterioration of the nervous system, but due to the involuntary movement of muscles can cause tendon contractures and require surgical treatment.
Pathogenesis
Gene linkage analysis and locational cloning found that EA1 was caused by mutations in the KCNA1 gene. This gene is located on 12p13 and encodes the voltage-gated potassium channel Kv1.1a subunit. Mice lacking this gene can show obvious neuromuscular conduction temperature sensitivity, and exhibit excessive excitement of the presynaptic membrane under tension, similar to the symptoms of human EA1.
Kv1 is a member of the voltage-gated potassium ion channel family. This channel is a glycosylated peptide complex composed of 4 α and β subunits, of which the a subunit is the main functional unit. The 4 ax subunits are arranged symmetrically around a center, forming a hydrophilic hole. Each a subunit contains 5 hydrophobic transmembrane spiral segments (S1-S3, S5-S6) and one hydrophilic transmembrane spiral segment (S4). The two transmembrane fragments are connected by peptide chains inside or outside the membrane. The peptide chain between S5 and S6 spirals into the lipid bilayer of the membrane to form the lining of the pores, which determines the selectivity of ion channels. S4 contains positively charged amino acids, which sense the ion gradient through the membrane. Kv1.1 is widely present in the central nervous system, especially the membrane specialized area of the initial segment of the cerebellar Purkinje cell axon and the proximal node area of the motor neuron axon. These ion channels are closed when the membrane potential is at rest, and open quickly when they are depolarized, forming most of the action potential repolarization.
The EA1 gene mutation changes the function of potassium channels through the following two mechanisms: 1. reducing the expression of potassium channels on the cell; 2. changing the gate voltage of potassium channel activation and inactivation. And put forward the following hypothesis: the potassium ion channel expressed by the gene mutation of EA1 can perform normal functions, and under certain conditions, exposure to certain molecular pathological changes (including unknown cytokines or ion channel modifiers) changes the potassium ion channel The function affects the activation and inactivation, and then affects the resting membrane potential, repolarization, the frequency of action potentials and the activity and function of other ion channels, causing a series of clinical symptoms.
EA2
Clinical Manifestations
This type of ataxia is often accompanied by interictal nystagmus, especially when gazing downward and outward. Seizures are often caused by mental stress, fatigue and exercise. Alcohol can sometimes be a precipitating factor and has nothing to do with shock. The onset lasts for several hours to--days or more. Attacks of ataxia are often accompanied by dizziness, headache, nausea and discomfort. It usually starts in childhood and lasts into adulthood. Some patients may have progressive ataxia and dysarthria, but the episodic symptoms of such patients are not obvious. The clinical manifestations of some cases are similar to spinocerebellar degeneration. Magnetic resonance imaging (MRI) showed atrophy of the cerebellar vermis. Positron emission tomography (PET) examination revealed that glucose metabolism in the entire cerebellum, anterior temporal lobe, and thalamus during the interictal period was reduced.
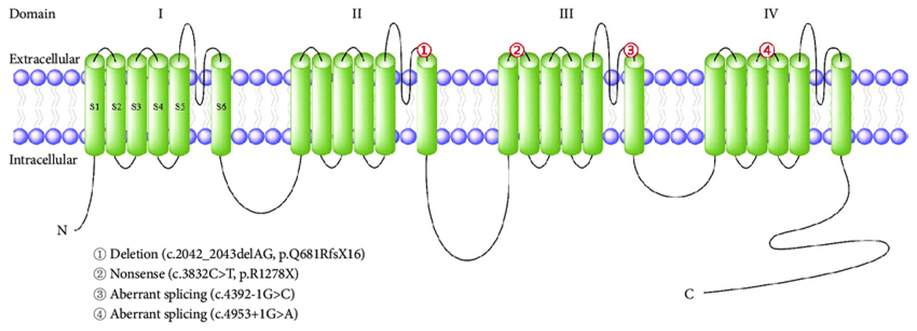
Figure 1. Literature reports of Korean families with episodic ataxia type 2.(Kwang-Dong Choi, et al.; 2016)
Pathogenesis
In 1996, Ophoff et al. discovered that EA2 was caused by mutations in the gene CACNA1A, which encodes the P/Q calcium channel Cav2.1α1 subunit. The gene is located at 19p13 and consists of 47 exons of about 300kb. Cav2.1 is composed of α1, α2, β, γ and δ subunits. α1: The subunit consists of 4 regions, each region is equivalent to a subunit of potassium channel. These 4 regions are arranged symmetrically to form calcium channel holes. P/Q type calcium ion channels are mainly expressed in cerebellar and brainstem neurons and the presynaptic membrane of neuromuscular junction. Its function is controlled by interacting with other receptor regulatory proteins and neurotransmitters. Calcium ion channel dysfunction makes calcium ions enter people abnormally, and eventually causes calcium accumulation in mitochondria, energy failure, neuronal death, such as cerebellar atrophy.
Paroxysmal Choreoathetosis with Paroxysmal Ataxia
In 1996, Auburger reported a family with paroxysmal choreotathotheliosis with paroxysmal ataxia. In the four generations of family members, there are 18 patients. Usually onset at the age of 2 to 15 years, mainly manifested as paroxysmal involuntary movement, postural dystonia of arms, legs and toes, balance disorders, dysarthria, paresthesias of the mouth and lower limbs, diplopia, sometimes accompanied by or Secondary headache. There was no obvious cerebellar ataxia during the attack. Physical exercise, emotional stress, lack of sleep and alcohol can aggravate attacks. The gene analysis of the family found that the gene mutation is located on 1p, in the 2cM region between DIS476 and D1S443, which is the gene encoding the potassium channel.
References
- Kwang-Dong Choi, et al.; Episodic Ataxias: Clinical and Genetic Features. Journal of Movement Disorders. 2016, 9(3):129-135.
- Kullmann DM, et al.; The inherited episodic ataxia: how well do we understand the disease mechanisms? Neuroscientist. 2001, 7(1):80-88.
- Pongs O. Voltage-gated potassium channels from hyperexcitability to excitement. FEBS Letters. 1999, 452:31-35.
- Waton JE, et al.; Integretion of high-resolution array comparative genomic hybridization analysis of chromosome 16q with expression array data refines common regions of loss at 16q23-qter and identifies underlying candidate tumor suppressor genes in prostate cancer. Oncogene. 2004, 23 (19): 3487-3489.
Related Section
Inquiry

