- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Long QT Syndrome
Long QT syndrome (LQTS) is a disease in which the heart repolarizes after a heartbeat. This can increase the risk of arrhythmia and may even cause fainting, drowning, seizures or sudden death. Among them, exercise or stress may trigger these attacks. Some rare forms of LQTS are related to other symptoms and signs, including periods of deafness and muscle weakness. Among patients, some are born with genetic mutations that cause long QT syndrome (congenital long QT syndrome), and some are caused by certain drugs, mineral imbalances, or medical conditions.
Hereditary or congenital long QT syndrome is caused by genetic abnormalities. LQTS may come from the variation of several genes, which can lead to completely different characteristics in different situations. It is worth noting that what the different mutants of LQTS have in common is that these mutations affect one or more ionic currents, which in turn leads to a prolonged ventricular action potential, which results in a prolonged QT interval. At present, a classification system has been proposed to distinguish the subtypes of the disease based on clinical characteristics and potential genetic variation. The most common case is Romano-Ward syndrome, which accounts for 99% of cases (usually types LQT1-6 and LQT9-16), an autosomal dominant form in which the electrical activity of the heart is affected, but no other organ. A less common form is Jervell and Lange-Nielsen syndrome, which is an autosomal recessive form of LQTS that combines prolonged QT interval and congenital deafness. Other rare forms include Anderson-Tawil syndrome (LQT7) and Timothy syndrome (LQT8). The former features prolonged QT interval, periodic paralysis, and facial and bone abnormalities; the latter manifests as prolonged QT interval and abnormal heart structure, and Autism spectrum disorder is related.
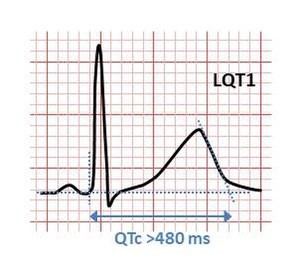
Figure 1.ECG of Long QT syndrome
The various forms of congenital and acquired long QT syndrome can produce abnormal heart rhythms (arrhythmia) by affecting the electrical signals that coordinate individual heart cells. The common feature is the prolonged cardiac action potential, which is the characteristic pattern of voltage changes on the cell membrane during each heartbeat. When relaxed, there are fewer positively charged particles on the inside of the heart cell membrane than on the outside, forming a polarized membrane. When heart cells contract, positively charged ions (such as sodium and calcium) enter the cell, making the polarity equal or opposite, thereby depolarizing the cell. After contraction, the cell restores its polarity (or re-polarizes) by allowing positively charged ions (such as potassium) to leave the cell to restore the membrane to its relaxed polarization state. In long QT syndrome, this repolarization takes longer to occur, which appears as a longer action potential in a single cell, and as a long QT interval on a surface electrocardiogram (ECG).
Prolonged action potentials can cause arrhythmia through a variety of mechanisms. The characteristic arrhythmia of long QT syndrome, that is, twisting, starts in the form of afterdepolarization the initial action potential and further triggers the abnormal action potential. Early depolarization occurs before the cell is completely repolarized, especially when the action potential is prolonged. This is caused by the reactivation of calcium and sodium channels, which are closed under normal physiological conditions until The next heartbeat is coming. Under the right conditions, the reactivation of these currents promoted by the sodium-calcium exchanger can lead to further depolarization of the cell. The arrhythmia induced by the early depolarization of long QT syndrome is caused by Purkinje fibers in the cardiac conduction system. Early depolarization may occur as a single event, but it may also occur repeatedly, leading to multiple rapid cell activation. Some studies have shown that delayed post-depolarization, that is, after repolarization is complete, may also be related to long QT syndrome. This form of post-depolarization originates from intracellular calcium ion storage, that is, the spontaneous release of the sarcoplasmic reticulum, forcing calcium ions to exchange sodium from outside the cell through a sodium-calcium exchanger, generating an inward net current.
References
- Tester DJ, et al. "Congenital Long QT Syndrome". In Gussak I, Antzelevitch C (eds.). Electrical Diseases of the Heart. London: Springer. 2013, pp. 439-468. doi:10.1007/978-1-4471-4881-4_27
- Wit AL, et al. "Afterdepolarizations and triggered activity as a mechanism for clinical arrhythmias". Pacing and Clinical Electrophysiology. 2018, 41 (8): 883-896. doi:10.1111/pace.13419
- Jáuregui-Garrido B, et al. Sudden death in eating disorders. Vascular Health and Risk Management. 2012, 8: 91-8.
- "Long QT syndrome". Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. 2017. Retrieved 14 December 2017.
Related Section
Inquiry

