- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Nonsyndromic Deafness
Deafness is the most common birth defect disease. Hereditary deafness can be divided into two types: one is syndromic hearing impairment (SHI) and the other is non-syndromic hearing impairment (NSHI), and 70% of hereditary deafness manifests as NSHI. Non-syndromic deafness is partial or total hearing loss unrelated to other signs and symptoms. Conversely, the symptoms and signs of syndrome deafness affect other parts of the body.
Non-syndromic hearing loss can be classified in several different ways. A common way is through the inheritance mode of conditions: autosomal dominant inheritance (DFNA), autosomal recessive inheritance (DFNB), X-linked (DFNX) or mitochondria. Each of these types of hearing loss includes multiple subtypes. Among them, the numbering sequence of DFNA, DFNB and DFNX subtypes is the sequence described for the first time.
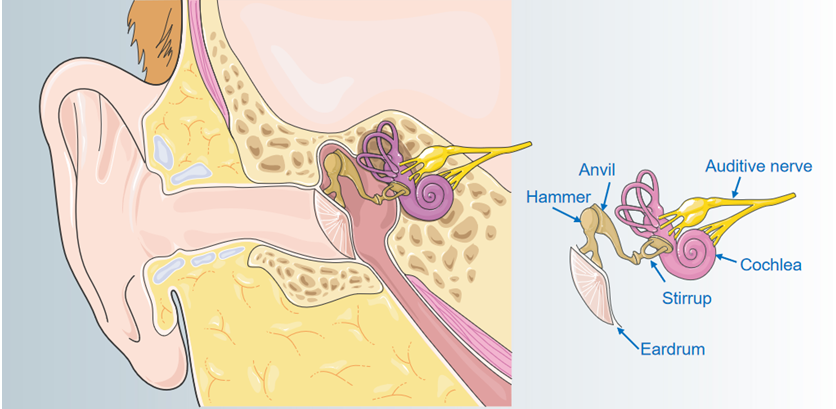
Figure 1. Internal structure of the ear.
The characteristics of non-syndromic deafness vary between different types. Hearing loss can affect one ear (unilateral) or both ears (bilateral). The degree of hearing loss ranges from mild (difficult to understand soft speech) to severe (even loud sounds). In clinical studies, it has been found that the degree of hearing loss in patients can be stable or gradual. Certain types of non-syndromic hearing loss exhibit unique hearing loss patterns. For example, at high, mid or bass, the loss may be more pronounced.
Most forms of non-syndromic hearing loss are described as sensorineural, which means they are related to permanent hearing loss caused by structural damage to the inner ear. The inner ear processes sound and sends information to the brain in the form of electrical nerve impulses. Less commonly, non-syndromic hearing loss is described as conductive, which means it is caused by changes in the middle ear. The middle ear contains three small bones that help to transmit sound from the tympanic membrane to the inner ear. Certain forms of non-syndromic hearing loss, especially the type called DFNX2, involve changes in the inner and middle ears. This combination is called mixed hearing loss.
Pathogenesis
Studies have shown that human NSHI is closely related to a few gene mutations, such as mtDNA 12 Sr-RNA, SLC26A4, GJB2, GJB3 and GJB6.
mtDNA 12 SrRNA Gene Mutation
Some mutations in the mitochondrial 12 SrRNA gene are closely related to the occurrence of non-syndromic deafness. The mtDNA mutations mainly occur in the 12 SrRNA gene and the tRNASer (UCN) gene, and some mutations in the 12 SrRNA gene are aminoglycoside antibiotic induced deafness (AAID) important molecular basis. At present, a large number of studies have confirmed that mtDNA mutations are closely related to AAID deafness, and some patients are susceptible to aminoglycoside drugs due to individual differences (that is, the application of normal doses or trace amounts of aminoglycoside drugs can induce hearing loss) . The main susceptibility factor of AAID is the A1555G mutation on the 12 SrRNA of the mitochondrial DNA gene, and the C1494T mutation and 961insC on the 12 SrRNA of the mitochondrial DNA gene can increase the risk of AAID caused by the A1555G mutation. Therefore, genetic testing and screening of deafness can effectively reduce the incidence of AAID. In addition, mitochondrial 12 SrRNA T1095C mutations have also been detected in patients with deafness that are associated with non-syndromic deafness.
SLC26A4 Gene Mutation
SLC26A4 gene is mainly expressed in the thyroid, inner ear, and kidney. This gene encodes Pendrin protein, which is related to the transport of chloride-iodide ions. The mutation spectrum of SLC26A4 gene is very wide, and more than 170 mutation modes have been discovered and reported. The mutation sites are mostly distributed in the coding region and splice site of SLC26A4, and the non-coding region exon 1 binding transcription regulator (FOXI1) promoter sequence.
GJB2 Gene Mutation
GJB2 is the first genetic deafness gene that has been cloned and identified, and it is also the most common pathogenic gene that causes NSHI. The GJB2 gene provides instructions for the synthesis of connexin 26, which is a member of the connexin family. There are more than 220 mutation modes, and the inheritance mode of deafness can be autosomal recessive inheritance and autosomal dominant inheritance. There are 3 most common mutation sites in GJB2, namely 35delG, 167delT and 235delC.
GJB3 Gene Mutation
GJB3 gene mutation is closely related to the mutation of connexin31 (Cx31) that it encodes. The mutation of the connexin gene causes potassium ions to flow back into the endolymph fluid, which affects the endolymph potential, resulting in abnormal hair cell function. GJB3 mutation can cause both autosomal dominant non-syndromic hearing loss and autosomal recessive non-syndromic hearing loss (DFNB).
GJB6 Gene Mutation
GJB6 is expressed in a variety of tissues and organs, such as inner ear, skin, and brain. Cx30 is a gap junction protein encoded by the GJB6 gene. Mutations in this gene are the main mutation mode for children with non-syndromic pre-linguistic deafness. GJB6 has 77% sequence homology with GJB2, but the C-terminal of GJB6 is 37 amino acids more than GJB2. The most common mutation of GJB6 in patients with non-syndromic deafness is the del (GJB6-D13S1830) homozygous deletion or the heterozygous deletion coexisting with the GJB2 monoallelic mutation.
At present, the incidence of genetic diseases and birth defect diseases is gradually increasing. In some economically developed countries and regions in the world, genetic diseases and congenital defect diseases are replacing infectious diseases, and they with vascular diseases and cancer have become the three diseases that seriously endanger human physical and mental health and have the highest mortality rate. Nowadays, although there are no effective treatment methods and methods for human genetic diseases and birth defect diseases, with the rapid development of molecular biology technology, it has been possible to carry out genetic screening for many genetic diseases and some birth defect diseases. Therefore, targeted genetic counseling for high-risk groups of such genetic diseases and pregnancy screening and prenatal diagnosis of high-risk fetuses can effectively prevent the birth of new children and improve population quality.
References
- Ding Y, et al.; The role of mitochondrial DNA mutations in hearing loss. Biochem Genet. 2013, 51(7-8):588-602. doi: 10.1007/s10528-013-9589-6.
- Duman D, Tekin M. Autosomal recessive nonsyndromic deafness genes: a review. Front Biosci (Landmark Ed). 2012, 17:2213-36.
Related Section
Inquiry

