- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
Patch-Clamp Recording Protocol
The major limitation of in vivo patch-clamp recording is its technical difficulty, which requires experienced personnel and a large amount of patience. The workflow of patch-clamp recording involves pressing a glass micropipette against a cell in order to isolate a small "patch" of membrane that contains one or more ion channels. The experimental setup further allows researchers to "clamp" the electrical environment of the patched area by precisely controlling the voltage across the cell membrane, which, depending on the ion channels present, impacts the flow of ions through the membrane and allow for intricate study of these channels.
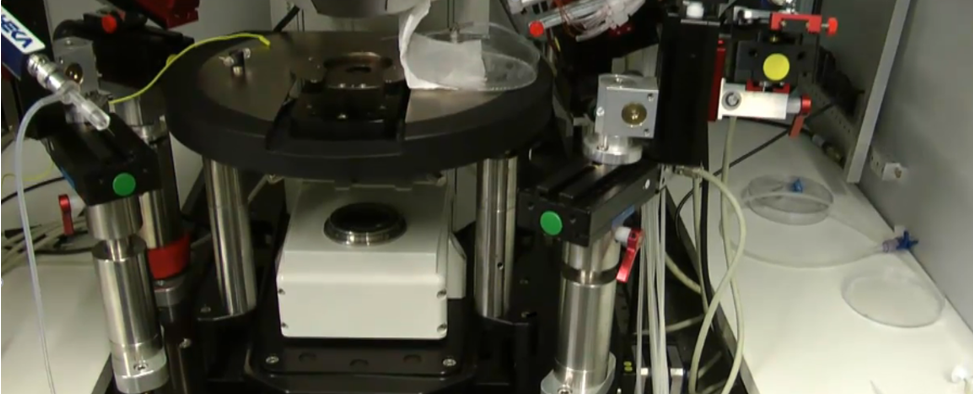
Here we go over the steps required to perform a patch clamp recording.
Start by pulling a borosilicate glass tube into micropipettes using a pipette puller.
Next, fire polish the tip to obtain the appropriate diameter and resistance.
After polishing, fill the micropipette with an ionic solution and flick gently to dislodge any air bubbles.
Then slide the micropipette over the electrode attached to a holder.
Once attached, use a syringe to apply positive pressure to the pipette, which prevents other solutions from entering the tip.
Place your cells or tissue of interest on the microscope stage and move the micropipette towards a cell.
With the amplifier generating test voltage pulses, record the resistance, which will increase once the tip is touching the cell.
To form the gigaohm seal, gently switch from positive to negative pressure using the syringe. The formation of the seal will result in a rapid increase in resistance to greater than 1 gigaohm.
Now that a cell-attached configuration has been established, and then convert to a whole-cell configuration when the membrane is ruptured. The technique is particularly applicable to small cells in the size range of 5‐20 µm in diameter, and yields good recordings in cells as small as red blood cells.
Rupturing is accomplished by adding negative pressure to the micropipette. To be more specific, negative pressure is applied to form a giga seal. Suction or a "zap" voltage pulse can be applied to break the membrane. A set of programmable valves control these pressure-switch operations.
Once the membrane breaks, the test pulse shape will have large current transients, as the cell membrane is now acting as a capacitor, which is charged by the test pulse.
The properties of a single ion channel type in any given neuron can be investigated by blocking the activity of other channels types pharmacologically.
A voltage-step protocol is used to examine ion channel currents evoked by stepping the voltage to a series of different holding potentials.
The current-voltage or IV curve shows the voltage-dependences of current flowing through an ion channel and provides insight into at which voltages the channel is open or closed.
Key Factors of a Successful Patch-Clamp Experiment
- Clean pipette tip
- The formation of a giga seal
- Proper positive pressure during penetration
- Successful membrane break-in
Expectations of Patch-Clamp Recording
It has been attracting more attention in the neuroscience field with electrophysiological techniques. Particularly for researchers who are interested in intact neural circuits, in vivo patch clamp recording could be very helpful if properly combined with other tools, such as optogenetics and two-photon imaging.
References
- Patch Clamp Electrophysiology, JoVE Science Education Library.
- Can Tao, et al. Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience. Front. Neural Circuits. 2015.
Related Section
Inquiry

