- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0120 | Human KCNJ11/ABCC9 Stable Cell Line-HEK293 | ABCC9 | Human | Epithelial | INQUIRY |
ABCC9 is a gene that directs sulfonylurea receptor 2 (SUR2) protein synthesis. This protein participates in the formation of a channel for transporting charged potassium ions across the cell membrane. Each channel consists of 8 subunits: 4 SUR2 proteins and 4 proteins produced by KCNJ8 or KCNJ11 genes. The SUR2 subunit regulates the activity of the channel and determines whether the channel is open or closed. The channel composed of SUR2 protein is called atp-sensitive potassium channel (K-ATP). The opening and closing of these channels depends on the amount of ATP, the main energy source in the cell. The resulting potassium ion transmission is part of a complex signal network that transmits chemical information to the inside and outside of the cell. Although K-ATP channels exist in cells and tissues throughout the body, the highest levels of channels containing sur2 in bone and heart muscle. These channels indirectly help regulate the concentration of calcium ions in cells. This regulation is essential for normal heart function. The function of these channels in other tissues is unclear.
The characteristic of ATP-sensitive potassium channels is that when the cell surface ATP concentration increases, the channel opening is inhibited. Studies have found that sulfonylurea drugs can inhibit the activity of K (ATP) channels. Therefore, such drugs are widely used as oral hypoglycemic agents for the treatment of non-insulin-dependent diabetes.
ABCC9 Clone and Structure
Aguilar-Bryan et al. cloned the cDNA corresponding to the high-affinity sulfonylurea receptor SUR. Further research found that the pancreatic β-cell K (ATP) channel consists of at least two subunits, BIR and SUR. The researchers cloned a subtype of SUR from the rat brain cDNA library and named it sulfonylurea receptor-2 (SUR2). This 5300 bp cDNA sequence encodes a polypeptide of 1,545 amino acids, which is 68% homologous to SUR. Northern blot analysis showed that the tissue distribution of Sur2 and SUR was different. Afterwards, the researchers used rat Sur2 as a probe to clone Sur2 from the skeletal muscle cDNA library. Northern blot detected three SUR2 transcripts, which were 9.4, 7.6, and 5.6 kb, and the longer transcripts in the heart and skeletal muscle had the highest levels. There is little or no expression of SUR2 in other tissues.
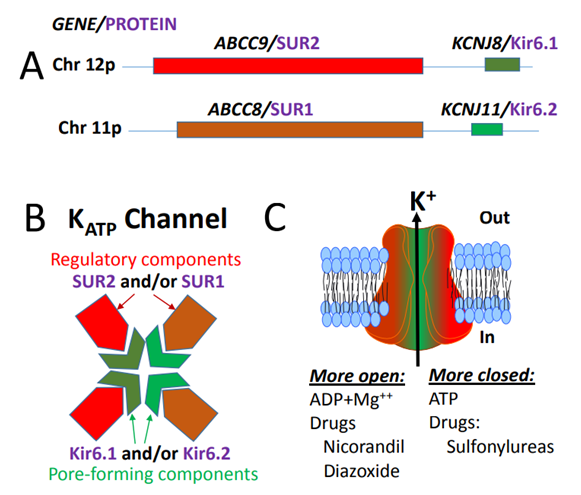
Figure 1. Schematic representation of the genes and proteins that make up the human KATP channel.(Peter T.Nelson, et al.; 2015)
ABCC9 and Disease
Cantú Syndrome
At least 14 mutations in the ABCC9 gene have been found to cause Cantú syndrome, a rare disease characterized by excessive hair, distinctive facial appearance, and heart defects. Each mutation changes a protein component of the SUR2 protein. These changes may change the structure of the protein and its ability to regulate the activity of K-ATP channels. Research has shown that abnormal channels are open when they should be closed. However, it is not clear how problems with potassium channel function lead to overgrowth of hair, heart defects, and other features of Cantú syndrome.
Dilated Cardiomyopathy
There are at least two mutations in the ABCC9 gene in patients with dilated cardiomyopathy. Dilated cardiomyopathy is a heart disease that enlarges and weakens the heart muscle and prevents the heart from pumping blood effectively. Symptoms and signs of this condition include irregular heartbeat (arrhythmia), shortness of breath, extreme fatigue (fatigue), and swelling of the legs and feet. Researchers have found two mutations in the patient's ABCC9 gene exon 38, leading to dilated cardiomyopathy. Existing studies have shown that the C-terminus of the SUR protein is involved in the transport of the K (ATP) channel. The researchers found that the translocation and missense SUR2A mutants recombined with Kir6.2 have reduced expression in the plasma membrane, while the mutant K (ATP) channel complex can form a functional channel with intact pore characteristics. Structural molecular dynamics stimulation showed that ala1513 and leu1524 residues are located on the side of the c-terminal β chain, close to the iconic Walker A motif, which is necessary for the coordination of nucleotides in the catalytic pocket of the atp binding cassette protein. Replacing ala1513 with threonine residues with larger space and stronger hydrophilicity or C-terminal truncation caused by frame shifting will disrupt the folding of the C-terminal beta chain. In the two channel mutants, the ATP-induced K (ATP) channel gating was abnormal, indicating that the mutant-induced structural changes distorted the ATP-dependent pore regulation.
References
- Bienengraeber, et al.; ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic K(ATP) channel gating. Nature Genet. 2004, 36: 382-387.
- Olson, T. M., et al.; K(ATP) channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4: 110-116.
- Bryan J, et al.; ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007, 453(5):703-18.
- Harakalova M, et al.; Dominant missense mutations in ABCC9 cause Cantú syndrome. Nat Genet. 2012, 44(7):793-6.
- Solbach TF, et al.; ATP-binding cassette transporters in the heart. Trends Cardiovasc Med. 2006, 16(1):7-15.
- Van Bon BW, et al.; Cantú syndrome is caused by mutations in ABCC9. Am J Hum Genet. 2012, 90(6):1094-101.
- Peter T.Nelson, et al.; ABCC9/SUR2 in the brain: Implications for hippocampal sclerosis of aging and a potential therapeutic target. Ageing Research Reviews. 2015, Volume 24, Part B, Pages 111-125.
Inquiry
