- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- asic2
asic2
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0064 | Human ACCN1 Stable Cell Line-CHO | ASIC2 | Human | Epithelial-like | INQUIRY |
ASIC2, also known as amiloride-sensitive cation channel 1, neuron (ACCN1) or brain sodium channel 1 (BNaC1), are acidic channels that are mainly involved in pH regulation in the body and are an important component for maintaining homeostasis. Existing research shows that ASICs are encoded by 4 functional genes, namely asic1, asic2, asic3, and asic4.
Discovery of ASIC2
In 1996, Price et al. cloned a new cDNA encoding a voltage-independent sodium channel from the human brain, which they called BNC1. Further research found that BNC1 has a certain sequence similarity with members of the amiloride sodium-sensitive sodium channel family, including mammalian epithelial sodium channels. However, among other differences, when the BNC1 channel was co-expressed with other cloned subunits of the family, its activity did not increase. Therefore, researchers believe that BNC1 is the first cloned member of the new subfamily of mammalian Na+ channels. Subsequently, only 2.7- and 3.7 kb transcripts were detected in the brain and spinal cord in Northern blot analysis, suggesting that BNC1 may play a role in neurotransmission. Later, Garcia-anoveros et al. isolated cDNAs encoding ACCN1 and ACCN2 and named them BNaC1 and BNaC2, respectively. The predicted identical rate of BNaC1 and BNaC2 proteins was 68%. Northern blot analysis and mouse brain in situ hybridization showed that these two genes are co-expressed in most brain neurons, and are expressed in early embryogenesis and throughout the life cycle. As the research progresses, scientists have used real-time fluorescent quantitative PCR to detect the expression of ASIC1 (ACCN2), ASIC2 and ASIC3 in human bone biopsy specimens. These ASICS are also expressed in cultured human monocytes and differentiated osteoclasts. Western blot analysis detected a 55kd ASIC2 protein in both the membrane and cytoplasm of cultured human osteoblasts. So far, multiple subunits of ASICs, ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4, have been cloned. Among them, ASIC2a and ASIC2b are variants from ACCN1.
ASIC2 Protein Structure
ASIC subunits are composed of two transmembrane (TM) domains, a large extracellular loop and short cytoplasmic N- and C-termini. ASIC2a and ASIC2b have different amino acid sequences at the N-terminus, TM1 domain and one-third of the extracellular loop region, while the remaining sequences are the same. Several studies have used the sequence differences between the two subunits to find possible proton binding sites in ASIC2a, and identified five amino acids (H72, D77, E78, H109 and H180) that are not present in ASIC2b as putative protons binding site.
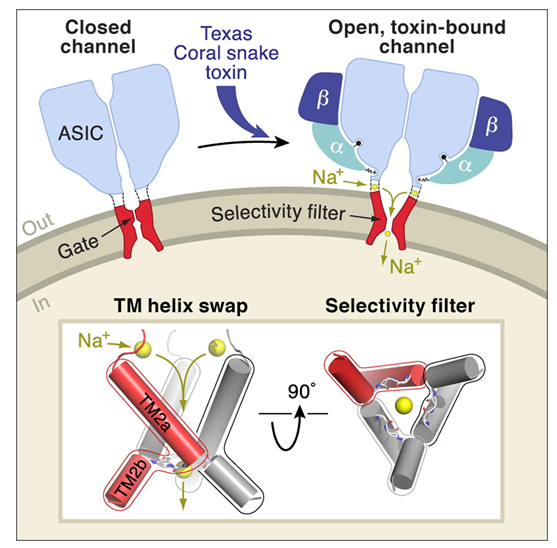
Figure 1. X-Ray Structure of Acid-Sensing Ion Channel 1. (Baconguis I, et al.; 2014)
ASIC2 Protein Distribution
In cultured hippocampal neurons, ASIC2a mainly displays somatic dendritic distribution in dendrites and dendritic spines. When ASIC2a is heterologously expressed in human embryonic kidney (HEK) 293 cells, it is mainly detected in the plasma membrane and other intracellular locations. However, ASIC2b is distributed in COS-7 cells in a mesh pattern. In vascular smooth muscle cells (VSMC), the ASIC2 protein shows a perinuclear staining pattern.
ASIC2 and Disease
Acidosis in the brain plays a key role in neuronal damage in neurological diseases, including cerebral ischemia. A key mediator of neuronal damage caused by acidosis is acid-sensitive ion channels (ASIC). At present, the main research object when studying acidosis signals in the brain is ASIC1a. Interestingly, studies have found that ASIC2 has a specific effect on the area of acid-mediated response. Deletion of ASIC2 reduced acid-activated currents in cortical and striatal neurons, but had no significant effect on cerebellar granule neurons. In addition, the researchers also discovered that ASIC2 is important for ASIC1a expression, and that ASIC2a also promotes ASIC1a surface transport in the brain. The absence of ASIC2 reduced neuronal damage caused by acidosis/ischemia in organotypic hippocampal slices. Therefore, the absence of ASIC2 in mouse brain studies showed significant protection in vivo from ischemic damage.
References
- Askwith CC, et al.; Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004, 279(18):18296-305.
- Baron, A., et al.; Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. 2001, 276, 35361–35367.
- Zha, X. M., et al.; ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. 2009, 29, 8438–8446.
- Chai, S., et al.; A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J. Biol. Chem. 2007, 282, 22668–22677.
- Grifoni, S. C., et al.; Hsc70 regulates cell surface ASIC2 expression and vascular smooth muscle cell migration. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2022–2030.
- Price, M. P., et al.; Cloning and expression of a novel human brain Na+ channel. J. Biol. Chem. 1996, 271: 7879-7882.
- Jahr, H., et al.; Identification of acid-sensing ion channels in bone. Biochem. Biophys. Res. Commun. 2005, 337: 349-354.
- Garcia-Anoveros, J., et al.; BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc. Nat. Acad. Sci. 1997, 94: 1459-1464.
- Baconguis I, et al.; X-ray structure of acid-sensing ion channel 1-snake toxin complex reveals open state of a Na(+)-selective channel. Cell. 2014, 156(4):717-29.
Inquiry
