- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0001 | Human CACNA1B Stable Cell Line-HEK293 | CACNA1B | Human | Epithelial | INQUIRY |
The voltage-dependent N-type calcium channel subunit α-1B (CACNA1B) is a protein encoded by the human CACNA1B gene. Together with the β and α2δ subunits, it forms an N-type calcium channel, namely the Ca v 2.2 channel. Therefore, the function of the CACNA1B protein is mainly achieved through the Ca v 2.2 channel.
N-type or CaV2.2 calcium channels belong to the family of high-pressure activated calcium channels. Nowycki, Fox and Tsien first discovered three types of Ca channels in the sensory neurons of the chicken dorsal root ganglia. In addition to the known L-type and T-type Ca channels, they also identified a third type of Ca channel, namely the N-type. Soon thereafter, Wanke et al. proved that the N-type channel in the upper cervical ganglion of rats is affected by the muscarinic pathway regulation of pertussis toxin-sensitive G protein.
CACNA1B Participates in Cav2.2 Channel Composition
In the early 1990s, scientists isolated and identified several clones of cDNAs encoding the y1 subunit homologous to the heart and bone subunits of pe1 from the rat brain; and then a 240 kDa n-type channel protein was detected and proved Its association with α2δ subtype. Westenbroek et al. discovered in the rat brain two α1B proteins with a size of 240 kDa and 210 kDa, which have local complementary effects in the dendrites, nerve endings and cell bodies of most neurons. Under normal physiological conditions, the α1B subunit mainly enhances the current through the channel complex by increasing the number of channels in its plasma membrane and its opening probability. The β subunit can regulate channel gating by changing the regulation of activation and inactivation.
The Role of CaV2.2 Channel in Physiology and Pathophysiology
Studies have found that the expression of CaV2.2 channels is almost exclusively in central and peripheral neurons. These channels are mainly located in the dendritic axis and presynaptic terminal. They are found in plates I and II in the dorsal horn, and are primarily responsible for transmitting nociceptive information to the synapses of the spinal cord. In the II-III domain linker of CaV2.2 α1 subunit, a synaptic protein interaction site was found. This site interacts with proteins that release complexes from synaptic vesicles, such as syntaxin 1 and SNAP-25. This interaction stabilizes the membrane positioning of the CaV2.2 channel near the synaptic vesicle. The afferent action potential activates a large amount of precise calcium influx through the CaV2.2 channel, and activates the release of neurotransmitters such as glutamate or substance P to ensure the transmission of peripheral signals to the brain. Transmit various sensory patterns of peripheral sensory neurons, This includes pain, itching, touch and feelings of muscle tension.
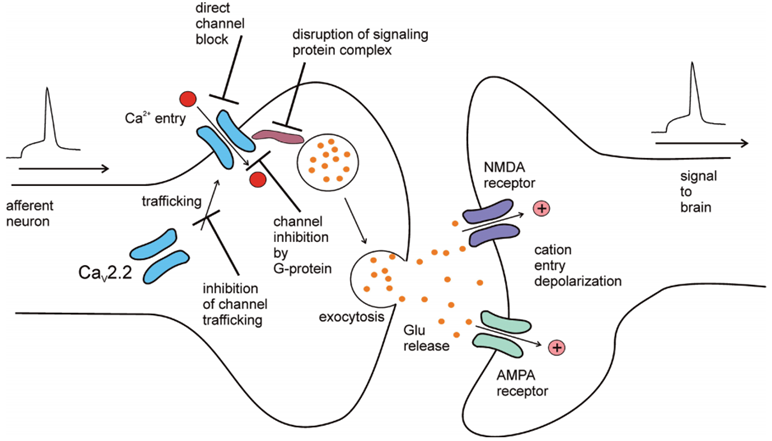
Figure 1. Role of CaV2.2 channels in synaptic transmission.(Jurkovicova-Tarabova B, et al.; 2019)
CaV2.2 Channel Regulation
- Regulation of G protein on CaV2.2 channel
G protein regulation of CaV2.2 channel may be the most studied CaV2.2 channel pathway regulation activity. G protein is a heterotrimer consisting of 3 subunits: α, ß, and γ. These intracellular complexes bind to the plasma membrane g protein coupled receptor (GPCR). Agonist activation of GPCR causes the dissociation of G protein heterotrimers into Gα and Gβγ subunits. The isolated subunit interacts with various effector molecules, including the α1 subunit of voltage-gated calcium channels. In the process of regulation, the Gβγ dimer directly binds to the α1 subunit of the CaV2.2 channel and initiates the transition of the gated state of the channel. The mechanism of regulating calcium entry through the CaV2.2 channel can regulate the release of neurotransmitters, thereby regulating the synaptic transmission of noxious signals by activating the synaptic GPRC.
- Regulation of CaV2.2 Channel by Protein Kinase
The CaV2.2 channel can be phosphorylated by the isoenzyme of protein kinase C (PKC). This phosphorylation leads to an increase in n-type calcium current, and this increase can be offset by an auxiliary β subunit. In resting sensory neurons, CaV2.2 channels can also be phosphorylated by calcium/calmodulin-dependent protein kinase II, and removal of this phosphorylation will result in loss of channel activity.
In short, the CaV2.2 channel plays a unique role in pain perception, and is an attractive target for the treatment of severe inflammation and/or neuropathic pain. Because blockers that directly act on channel proteins cannot pass through the blood-brain barrier and are blocked, understanding the complex signal network that regulates CaV2.2 channels may be a promising new drug design method.
References
- Jurkovicova-Tarabova B, et al.; Structure, function and regulation of CaV2.2 N-type calcium channels. Gen. Physiol. Biophys. 2019, 38(2), 101-110.
- Agler HL, et al.; G protein-gated inhibitory module of N-type (Cav2.2) Ca2+ channels. Neuron. 2005, 46: 891-904.
- Bourinet E, et al.; Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94: 81-140.
Inquiry
