- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- glra1
glra1
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0010 | Human GLRA1 Stable Cell Line-HEK293 | GLRA1 | Human | Epithelial | INQUIRY |
The glycine receptor (GlyR) is a member of the cys-loop family of ligand-gated ion channels and is responsible for mediating the inhibitory effect of glycine. They are widely distributed throughout the central nervous system, especially in the hippocampus, spinal cord and brainstem
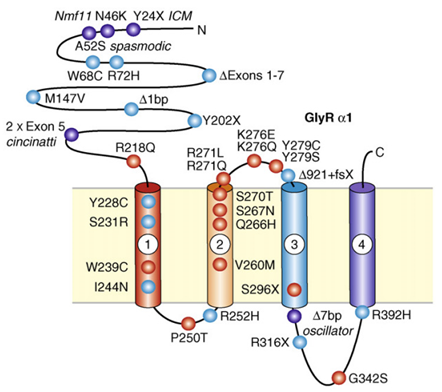
Figure 1. This schematic diagram depicts the suggested four-membrane spanning domain topology of GlyR a1. (Robert J. Harvey, et al.; 2008)
GLRA1 Function
The GLRA1 gene provides instructions for the synthesis of the α1 subunit of the glycine receptor protein. Existing studies have shown that the glycine receptor is composed of five subunits: two α1 subunits and three β subunits. Beta subunits are produced by different genes.
Glycine receptors are embedded in the membranes of nerve cells (neurons) in the spinal cord and the part of the brain that connects to the spinal cord (brain stem). Receptor proteins usually have specific positions on the membrane, and the corresponding molecules called ligands can enter these positions like a key and lock. Ligands and their receptors combine to trigger signals that affect cell development and function, and transmit them to the cell. The ligand of the glycine receptor is a component of protein(glycine). This molecule also acts as a neurotransmitter, a chemical messenger that transmits signals in the nervous system.
When glycine attaches (binds) to the glycine receptor, the receptor opens, allowing negatively charged chlorine atoms (chloride ions) to enter the neuron. The influx of chloride ions reduces the ability of neurons to transmit signals to other neurons. Because glycine receptors inhibit neuronal signal transduction, glycine receptors are called inhibitory receptors.
GLRA1 and Disease
Clinical studies have shown that the diseases associated with GLRA1 is Hereditary epithelial tics. The disease-related pathways include ligand gate ion channel transport and the transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds. This process involves the gene activity of GLRA1, including extracellular ligand-gated ion channel activity and extracellular glycine-gated chloride ion channel activity.
Hereditary Epithelial Tics
Studies have found that more than 60 mutations in the GLRA1 gene have been found to cause hereditary neurosis. This symptom is most common in infants, mainly through their experience of increased muscle tone (high tension) and an exaggerated startle response to unexpected stimuli, especially loud noises. The startle reaction can cause a brief period of stiffness and immobility, and in some severe cases, the baby will stop breathing. Most GLRA1 gene mutations change a single amino acid in the α1 subunit of the glycine receptor protein. The most common mutation is to replace the amino acid arginine with the amino acid leucine at position 271 of the protein.
Mutations in the GLRA1 gene cause hereditary epithelial tics, which impairs the ability of the glycine receptor protein to respond to the ligand glycine. Certain mutations in the GLRA1 gene change the structure of the glycine receptor, which prevents the receptor from opening or causes it to open in the absence of glycine. Other mutations prevent the receptor from reaching the cell membrane. When the glycine receptor functions abnormally or is absent, the unwanted chloride ions enter the cell or cannot enter the cell at all. Increased cell signal transduction in the spinal cord and brain stem may lead to abnormal muscle movements, exaggerated startle responses, and other signs and symptoms of hereditary epithelial nerve excess.
References
- Bode A, et al.; New hyperekplexia mutations provide insight into glycine receptor assembly, trafficking, and activation mechanisms. J Biol Chem. 2013, 288(47):33745-59.
- Harvey RJ, et al.; The genetics of hyperekplexia: more than startle! Trends Genet. 2008, 24(9):439-47.
- Masri A, et al.; Hyperekplexia: Report on phenotype and genotype of 16 Jordanian patients. Brain Dev. 2017, 39(4):306-311.
- Villmann C, et al.; Recessive hyperekplexia mutations of the glycine receptor alpha1 subunit affect cell surface integration and stability. J Neurochem. 2009, 111(3):837-47.
- Zhang Y, et al.; Investigating the Mechanism by Which Gain-of-function Mutations to the α1 Glycine Receptor Cause Hyperekplexia. J Biol Chem. 2016, 291(29):15332-41.
- Robert J. Harvey, et al.; The genetics of hyperekplexia: more than startle!. Trends in Genetics. 2008, 24(9), 439-447.
Inquiry
