- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0176 | Human GRIN1/GRIN2C Stable Cell Line-HEK293 | GRIN2C | Human | Epithelial | INQUIRY |
Existing studies have shown that NMDA receptors are a type of ionotropic glutamate receptors. Structural analysis revealed that the NMDA receptor channel is composed of the key receptor subunit GRIN1 and one or more isomers of the four NMDAR2 subunits (GRIN2A, GRIN2B, GRIN2C, and GRIN2D). In 1996, scientists screened the cDNA library of human hippocampal nerve cells by using the cDNA probe of rat GRIN2A, and isolated the cDNA encoding GRIN2C. Further research found that GRIN2C mRNA with a length of 4.4kb is widely expressed in the brain, especially in the cerebellum. Transcripts of this gene have also been found in several other tissues. In addition, another slightly larger transcript was found in the cerebellum. Further comparison found that the 1,233 amino acid protein sequence of the transcript has 88% identity with the rat and mouse GRIN2C sequences. The prediction of the hydrophilicity of GRIN2C showed that the GRIN2C protein contains a large N-terminus, 4 hydrophobic regions and a large C-terminus. Fluorescence in situ hybridization and somatic hybridization PCR technology were used to locate the gene of human NMDA receptor channel epsilon-3 subunit to 17q25.
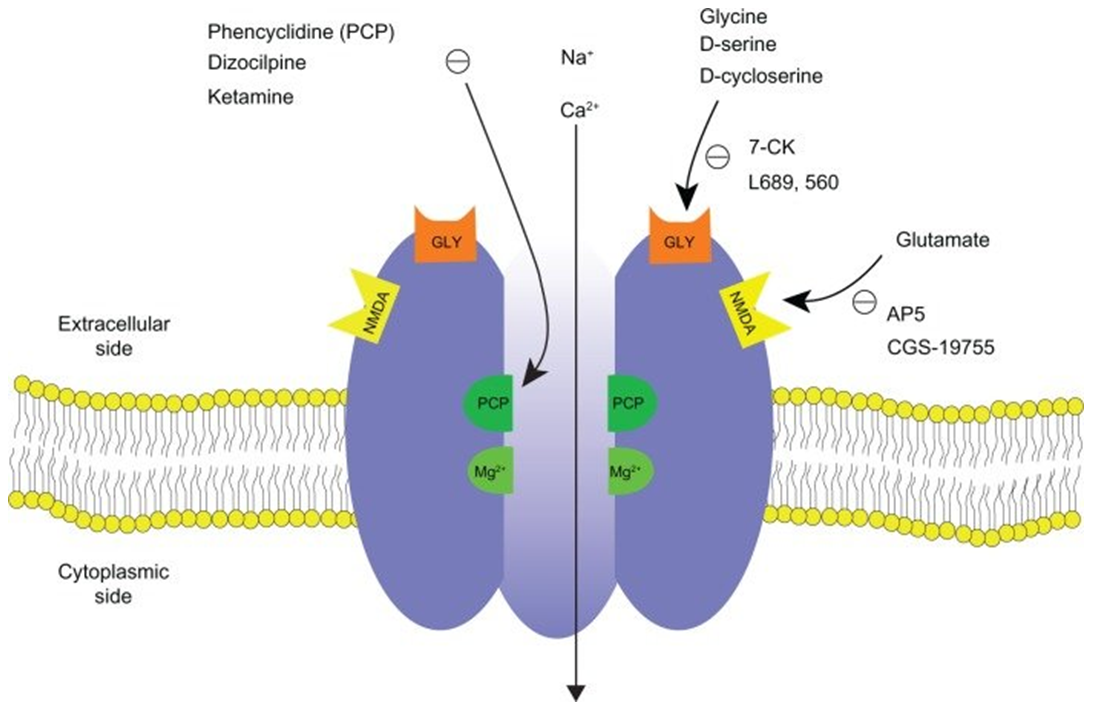
Figure 1. Schematic diagram of NMDA receptor complex. (Jih-Heng Li, et al.; 2011)
Biochemical Characteristics
The subunit-specific gating of NMDA receptors (NMDARs) is controlled by the region formed by the N-terminal domain (NTD) of NR2. NTD is an extracellular clamshell domain that binds to allosteric inhibitors, while short-chain linking NTDs are agonistic Agent binding domain (ABD). The subtype specificity of the maximum open probability (PO) of NMDAR largely reflects the difference in the spontaneous (ligand-independent) balance between the cleaved and closed cleaved conformations of NR2 NTD. In addition, NTD-driven gating control can also affect the pharmacological properties by setting the sensitivity to the endogenous inhibitor zinc and protons.
Gene Function
Existing studies have found that synapses and extra-synaptic NMDA receptors have opposite effects on CREB (cAMP response element-binding protein) function, gene regulation, and neuron survival. Among them, the entry of calcium through synaptic NMDA receptors is as strong as in inducing CREB activity and brain-derived neurotrophic factor (BDNF) gene expression as stimulating L-type calcium channels. On the contrary, the entry of calcium through extrasynaptic NMDA receptors triggered by glutamate exposure or hypoxia/ischemia activates a universal and dominant CREB blocking pathway, thereby blocking the induction of BDNF expression. Further studies have found that synaptic NMDA receptors have anti-apoptotic activity, and stimulation of extra-synaptic NMDA receptors leads to loss of mitochondrial membrane potential (an early marker of glutamate-induced neuronal damage) and cell death.
Ozaki et al. found that the neuregulin-β isoform (NRG1) increased the expression of the NR2C subunit of the NMDA receptor in cultured mouse cerebellar slices, and this upregulation also requires the synaptic activity of the NMDA receptor. These findings indicate that neuregulin regulates the composition of neurotransmitter receptors in the mature synapses of the brain.
Homozygous GRIN2C-deficient mice were produced by targeted destruction of the mouse GRIN2C gene. After observation, this mutant mouse has no obvious defects. After that, the scientists used gene targeting to generate mutant mice that express the NMDAR2C gene without a large intracellular C-terminal domain. The results indicate that these mice are viable, but exhibit deficits in motor coordination. Therefore, the phenotype observed for GRIN2C gene defects seems to reflect defective intracellular signaling.
References
- Gielen, M., et al.; Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009, 459: 703-707.
- Hardingham, G. E., et al.; Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 2002, 5: 405-414.
- Lin, Y. J., et al.; Cloning of the cDNA for the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery. Molec. Brain Res. 1996, 43: 57-64.
- Ozaki, M., et al.; Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997, 390: 691-694.
- Sprengel, R., et al.; Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998, 92: 279-289.
- Jih-Heng Li, et al.; To use or not to use: an update on licit and illicit ketamine use. Substance Abuse and Rehabilitation. 2011, 2(2):11-20.
Inquiry
