- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- grin2d
grin2d
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0177 | Human GRIN1/GRIN2D Stable Cell Line-HEK293 | GRIN2D | Human | Epithelial | INQUIRY |
Neuronal signals caused by glutamate are processed by the ionotype and metabolite subtypes of the receptor. The ionotropic receptor group contains a complete cation-specific ion channel, and is further subdivided into two types: N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors. The NMDA receptor channel is a heteropolymer composed of the key receptor subunit NMDAR1 (GRIN1) and 4 NMDAR2 subunits (GRIN2A, GRIN2B, GRIN2C and GRIN2D). GRIN2D is the coding component that composes the NMDA receptor channel. Its function is also closely related to the NMDA receptor channel.
GRIN2D Cloning and Expression
In 1998, Hess et al. used NMDAR2A cDNA to screen a human fetal brain cDNA library for research and successfully isolated the cDNA encoding GRIN2D. The predicted sequence of 1,336 amino acid human NMDAR2D protein has 95% identity with the sequence of rat Nmdar2d. Interestingly, the NMDAR1/GRIN2D receptor expressed in Xenopus oocytes and mammalian cells has different pharmacological and biophysical properties from other human recombinant NMDA receptors, indicating that the receptor functions have species characteristics.
GRIN2D Gene Function
N-methyl-D-aspartate (NMDA) receptors are a type of ionotropic glutamate receptors. NMDA channels have been shown to participate in the activity-dependent increase in the efficiency of long-term synaptic transmission, which is considered to be the basis of certain memory and learning.
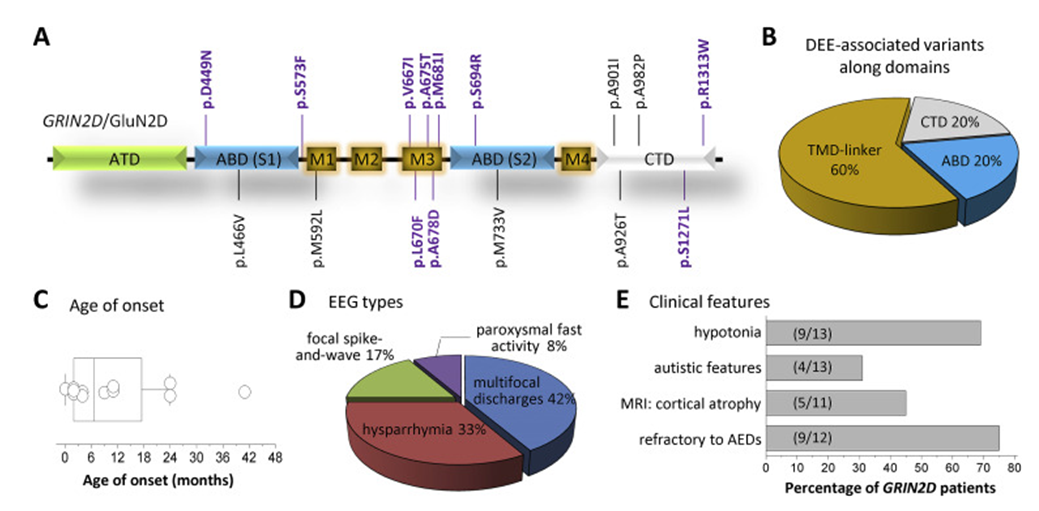
Figure 1. GRIN2D/GluN2D NMDA receptor.
Studies have found that calcium induces CREB activity and brain-derived neurotrophic factor (BDNF) gene expression through synaptic NMDA receptors as strong as the stimulation of type 1 calcium channels. In contrast, calcium entry through extrasynaptic NMDA receptors triggered by glutamate exposure or hypoxia/ischemia activates a universal and dominant CREB blocking pathway, thereby blocking the induction of BDNF expression. Synaptic NMDA receptors have anti-apoptotic activity, and stimulation of extra-synaptic NMDA receptors leads to loss of mitochondrial membrane potential (an early marker of glutamate-induced neuronal damage) and cell death.
GRIN2D and Disease
Developmental and epileptic encephalopathy 46
Developmental and epileptic encephalopathy-46 is a neurological disorder characterized by intractable seizures in the first few months or years of life. Affected individuals exhibited overall developmental delay, development failure, hypotonia, and hyperreflexia, accompanied by varying degrees of impaired mental development. More severe patients have little developmental progress and cannot sit or talk. In two unrelated girls with developmental and epileptic encephalopathy-46, the researchers identified a de novo heterozygous mutation in the GRIN2D gene. This mutation resulted in a V667I mutation at a highly conserved residue in the M3 transmembrane domain, which then replaced the core of the ion channel. Voltage clamp studies in transfected Xenopus oocytes and HEK293 cells showed that the mutation increased the receptor's response to glutamate and glycine agonists and reduced the channel's sensitivity to negative allosteric modulators. The inactivation time is prolonged and the possibility of channel opening is increased.
In addition, the researchers also identified in patients respectively that a mutation (M681I) occurred in the highly conserved residue of M3 in the GRIN2D gene, which changed the transmembrane domain formed by the channel; A S694R substitution occurred at a highly conserved residue in a ligand, which affected the binding domain of the channel; and a D449N substitution occurred at a highly conserved residue in the ligand, which changed the binding domain of the channel. These mutations all cause changes in the function of GRIN2D, which in turn leads to diseases.
References
- Gielen, M., et al.; Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009, 459:703-707.
- Hardingham, G. E., et al.; Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 2002, 5: 405-414.
- Hess, S. D., et al.; Functional characterization of human N-methyl-D-aspartate subtype 1A/2D receptors. J. Neurochem. 1998, 70: 1269-1279.
- Ikeda, K., et al.; Reduced spontaneous activity of mice defective in the epsilon 4 subunit of the NMDA receptor channel. Brain Res. Molec. Brain Res.1995, 33: 61-71.
- Kalsi, G., et al.; Localization of the human NMDAR2D receptor subunit gene (GRIN2D) to 19q13.1-qter, the NMDAR2A subunit gene to 16p13.2 (GRIN2A), and the NMDAR2C subunit gene (GRIN2C) to 17q24-q25 using somatic cell hybrid and radiation hybrid mapping panels. Genomics. 1998, 47: 423-425.
- Li, D., et al.; GRIN2D recurrent de novo dominant mutation causes a severe epileptic encephalopathy treatable with NMDA receptor channel blockers. Am. J. Hum. Genet. 2016, 99: 802-816.
- Chad R.Camp, et al.; GRIN2D/GluN2D NMDA receptor: Unique features and its contribution to pediatric developmental and epileptic encephalopathy. European Journal of Paediatric Neurology. 2020, Pages 89-99.
Inquiry
