- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- HCN1
HCN1
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0011 | Human HCN1 Stable Cell Line-HEK293 | HCN1 | Human | Epithelial | INQUIRY |
| ACC-RI0099 | Human HCN1 Stable Cell Line-HEK293, tetracycline-inducible | HCN1 | Human | Epithelial | INQUIRY |
In the past decade, hyperpolarized activated cyclic nucleotide gated (HCN) channels have attracted attention as a key factor in controlling and promoting neuronal excitability. Studies have found that the inward current Ih formed by the Na+ / K+ flowing during the opening of HCN seems to contribute to the spontaneous or ectopic discharge of various tissues including the central nervous system, peripheral ganglia and nerves. Due to the potential pathophysiological significance, the most interesting are those nociceptive neurons whose bodies are located in the dorsal root ganglia (DRG). Recent research results prove that Ih activation plays a role in promoting neuropathic pain (neuropathic pain is a poorly treated disease that requires new pharmacological strategies).
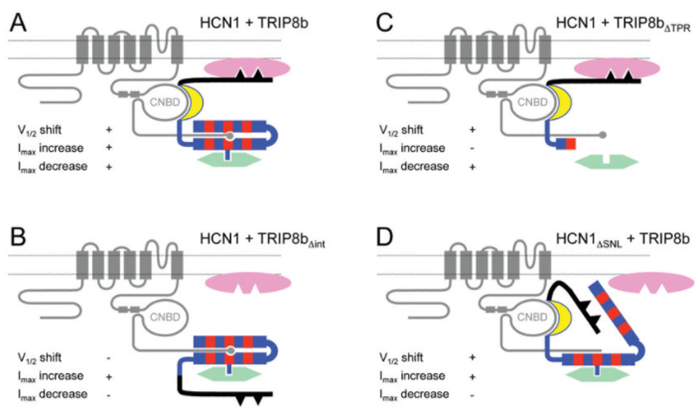
Figure 1. Schematic representation of HCN1/TRIP8b interactions in the presence of intracellular trafficking factors A. (Bina Santoro, et al.; 2011)
Studies have found that the HCN family is composed of four main isoforms (HCN1-4), which are assembled into homotetramers or heterotetramers in immature channels. And their biophysical properties and tissue distribution are significant difference in the body. Among them, HCN1 is mainly expressed in large neurons, HCN2 is dominant in small and medium neurons, and HCN3 and HCN4 are almost absent in all neurons. Structurally, the HCN channel is similar to the K+ channel, consisting of four domains, and each domain is composed of six transmembrane segments. In each domain, the S4 segment is positively charged and represents a voltage sensor. As the name suggests, these channels are activated by membrane hyperpolarization. Once opened, these channels will pass through Na+ and K+, which will lead to membrane depolarization. Their voltage sensitivity can be adjusted by cyclic adenosine monophosphate (cAMP) binding to the C-terminal site, which affects the voltage dependence of activation. This channel was first found in cardiac sinoatrial node cells, and its activation drives the pacemaker cells to the threshold voltage, activates the Ca2+ channel and activates the action potential. In the brain, HCN channels are expressed in the dendrites, somatic cells and axon ends of neurons, and they may produce rhythmic activity, thereby regulating the excitability of the network. Therefore, the role of HCN in many neurological diseases including epilepsy is accumulating.
Clinical Research of HCN1
HCN1 and Neuropathic Pain
Some people have recently demonstrated the feasibility of an anti-nociceptive strategy based on isoform-selective HCN blockers in a rat model of neuropathic pain. These results were obtained by using MEL57A, which is more selective for HCN1 than HCN2 and HCN4. The study found that the naïve Ih current recorded in DGR neurons is preferentially reduced by this HCN1 selective blocker. However, due to the related functions of HCN2 isoforms in the transmission of painful stimuli and the possibility of co-assembly of HCN1/HCN2 in heterotetramers, the strategy based on HCN1/HCN2 blockade (but not HCN4 blockade) may be an anti- A promising option for neuropathic pain.
HCN1 and Depression
Genetic studies of HCN channels in patients with severe depression have not yet found a close association between single nucleotide polymorphisms in the HCN gene and depression. However, researchers have developed animal models that selectively knock out HCN1, HCN2, or the Rab8b interacting protein (TRIP8b) (a brain-specific protein). Eliminating the expression of TRIP8b can cause h current to decay. In the HCN1, HCN2, and TRIP8b knockout strains, Ih dysfunction was associated with depression-related behaviors, as evidenced by the reduced immobility time in forced swimming and tail suspension tests. In addition, hippocampal HCN1 is related to the rapid antidepressant effect of ketamine, suggesting that HCN is a potential target for rapid treatment of depression.
HCN1 and Epilepsy
Initial research suggests that HCN channels seem unlikely to be candidates for pathogenic roles in epilepsy. However, recent evidence suggests abnormal functioning of these channels in hereditary and acquired epilepsy. It is not clear whether the knockout of the HCN1 channel also produces an epileptic phenotype, although anecdotal evidence suggests that HCN1 knockout mice exhibit spontaneous seizures, but show that more severe seizures are caused by pilocarpine. Studies in rat models have shown that the HCN1 gene has a key role in epilepsy, but there is a lack of clinical evidence that HCN1 mutations are involved in human epilepsy. So far, no human hereditary epilepsy lineage is associated with mutations in the HCN channel. However, more and more evidences indicate that acquired experimental epilepsy is related to changes in the function and expression of HCN channels.
References
- Dini L, et al.; Selective Blockade of HCN1/HCN2 Channels as a Potential Pharmacological Strategy Against Pain. Front Pharmacol. 2018 Nov 8;9:1252. doi: 10.3389/fphar.2018.01252. PMID: 30467478; PMCID: PMC6237106.
- Ming-HuHan, et al.; Neurobiology of Depression. Chapter 12 - Molecular, Cellular, and Circuit Basis of Depression Susceptibility and Resilience. 2019, Pages 123-136.
- B.S.Barker, et al.; Conn's Translational Neuroscience. Chapter 2 - Ion Channels. 2017, Pages 11-43.
- Bina Santoro, et al.; TRIP8b Regulates HCN1 Channel Trafficking and Gating through Two Distinct C-Terminal Interaction Sites. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011, 31(11):4074-86.
Inquiry
