- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0013 | Human HCN3 Stable Cell Line-HEK293 | HCN3 | Human | Epithelial | INQUIRY |
Hyperpolarized activated cyclic nucleotide-gated cation channels (HCN) are divided into four subtypes: HCN1, HCN2, HCN3 and HCN4. Unlike most voltage-gated channels, the activation of HCN channels is affected by a dual mechanism, namely voltage gating and ligand gating. The HCN channel of the cell membrane can be activated in a hyperpolarized state, and cAMP can directly bind to the channel protein on the cell membrane to activate the HCN channel and play an active role. In different cells, the activation voltage of HCN channels will also be different. Normally, the cell membrane can be activated at 50-60mV, which is close to the resting potential of most cells, and a hyperpolarization voltage of about 100mV can fully activate the HCN channel. At the same time, in the open state, the channel allows Na+ and K+ to pass through the cell membrane in a ratio of 1:3 to 1:5, showing non-selective permeability, forming an inward cation current, which is called Ih in the nervous system. And Ih is involved in many physiological processes of brain cognition, such as sleep, learning and memory. Since the discovery of the HCN channel, its hyperpolarization activation characteristics immediately attracted the attention of scientific researchers. HCN channels are involved in many neural activities, including the regulation of the rhythmicity of neuronal activity and dendritic integration, the regulation of cell resting membrane potential and synaptic transmission, and so on.
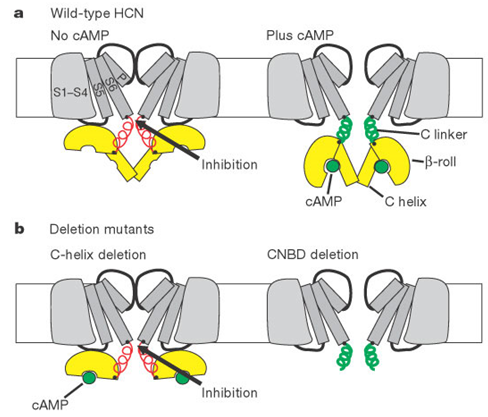
Figure 1. Model for cAMP modulation of HCN channel gating.(Brian J. Wainger, et al.; 2001)
Among them, Ih plays an important role in regulating the internal excitability and synaptic transmission of neurons. It can not only promote the proliferation of embryonic stem cells, but also process the optical feedback signals of the outer retina. In addition, studies have found that the postsynaptic current Ih in the globus pallidus can inhibit glutamatergic and GABAergic synaptic transmission, which indicates that HCN channels can regulate neurotransmitter release. In recent years, many researchers have studied the NMDA and GABAA receptors on the synapse and the amino acid transmitter receptors outside the synapse as the seizure mechanism involved in epilepsy diseases, and explored the role of HCN channels in epilepsy diseases. At the same time, some studies have shown that the abnormal regulation of HCN channels may be related to many neurological diseases, such as epilepsy, neuropathic pain, Parkinson's syndrome and so on.
In scientific research, it is found that HCN3 is less expressed, which makes channel research more difficult. For example, the expression of mouse HCN3 in HEK293 cells or similar systems results in very small and unstable currents. Until recently, the expression of murine HCN3 channels was increased through a lentivirus-based method. Therefore, compared with other HCN channels, information about the basic biophysical and pharmacological properties of HCN3 channels is limited. Existing studies have shown that HCN3 is less distributed in the central nervous system, and is more expressed only in the olfactory bulb, piriform cortex, preoptic area, habenula and some hypothalamic nuclei. Further studies have found that the increased distribution of HCN3 on the output neurons of the basal ganglia may be the mechanism leading to changes in the electrical activity of the output neurons of the basal ganglia in Parkinson's patients.
In order to explore the function of HCN3, scientists have cloned the HCN3 channel from the human brain to characterize the HCN3 channel in more detail. Further studies have found that the overall expression level of HCN3 in the human brain is low-only by dot blot analysis in neuronal tissue Human HCN3 mRNA was detected in. The highest expression level is found in the fetal brain. Compared with the expression of human HCN1 mRNA in brain tissue, the overall expression level is relatively low. Since HCN1 and HCN3 are potential pacemaker channels, human heart tissue are also detected. However, neither HCN1 nor HCN3 mRNA was detected in the human heart.
The human HCN3 channel is proven to be a fully functional, hyperpolarized activated cation channel with activation kinetics, voltage dependence and ion selectivity, as well as the sensitivity of cesium and ZD7288 in the range of other HCN channels. The significant difference from other hHCNs is that hHCN3 channels are not regulated by intracellular cAMP. The direct regulation of activation kinetics by cAMP is a distinctive feature of the other three HCN channels. The mechanism of cAMP modulation has been partially clarified. The HCN channel has a cyclic nucleotide binding domain located at the C-terminus of the cell. The C-terminus inhibits channel activation through an unknown interaction with the core region. When combined with cAMP, this inhibitory effect is released, resulting in less energy required for channel activation, resulting in faster activation and shifting activation to a higher positive activation potential. cAMP-dependent regulation is best observed for hHCN2 and hHCN4, while hHCN1 is only moderately regulated. It must be pointed out that in the absence of cAMP, the voltage-dependent activation curve of hHCN1 is already 20mV higher than the positive potential of hHCN2/4, which indicates that the inhibitory effect of the C-terminal is much smaller at first. Therefore, the release of inhibition The effect is not obvious. The activation curve of hHCN3 is close to that of hHCN1 channel, indicating that the inhibitory effect of the C-terminal is weak.
The reason for the lack of cAMP response in the hHCN3 channel is unknown. There are single amino acid differences scattered throughout the core region of the HCN channel. The importance of these differences is unclear. The study found that hHCN3 has the shortest C-terminal of all HCNs. This may result in less basic suppression. The short "inappropriate" C terminal does not seem to be able to control channel activation. Like the distal C-terminus, the N-termini of all HCNs are also significantly different from each other. However, sequences with definite functions, such as those identified as important for the assembly of the four HCN monomers to form a functional channel, remain in hHCN3. Therefore, it is possible that in natural tissues, hHCN3 forms heterotetrameric HCN channels, while other HCN subtypes contribute to cAMP sensitivity. Taking into account the basic characteristics, the hHCN3 channel will play the physiological role of the HCN channel, which is the stabilization of the resting membrane potential of the cell through the tonic effect of the inward current. Even the contribution to the neuronal pacemaker potential, which requires the synergy of many ion channels (including adaptive ion flow through HCN channels) does not depend on cAMP modulation. However, cAMP-mediated current modulation greatly expands the functional range of HCN channels. Therefore, the challenge remains to reveal the physiological role of HCN3 in the brain.
References
- Juliane Stieber, et al.; Functional Expression of the Human HCN3 Channel. J. Biol. Chem. 2005, 280:34635-34643.
- He C, et al.; Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014, 112:1-23.
- Della Santina L, et al.; Processing of retinal signals in normal and HCN deficient mice. PloS One. 2012, 7: e29812.
- Lason W, et al.; Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol Rep. 2013, 65:787-800.
- Brian J. Wainger, et al.; Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001, 411(6839):805-10.
Inquiry
