- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0035 | Human KCNB1 Stable Cell Line-CHO | KCNB1 | Human | Epithelial-like | INQUIRY |
Potassium voltage-gated channel, Shab-related subfamily, member1, also known as KCNB1 or KV2.1, is a protein encoded by the KCNB1 gene in humans. In mammalian CNS neurons, Kv2.1 is a major delayed rectifier K+ current that modulates neuronal excitability, action potential duration, and tonic spikes. In Drosophila photoreceptors, Kv.2 channels are key components of light-induced membrane voltage responses. Kv2.1 is widely expressed in the brain, constituting the majority of delayed rectifier K+ currents in cortical and hippocampal pyramidal neurons, and is also widely expressed in interneurons. Dynamic regulation of Kv2.1 localization and function through a mechanism involving activity-dependent Kv2.1 dephosphorylation significantly affects the intrinsic excitability of neurons.
Kv2.1 Structure
The Kv2.1 alpha subunit polypeptide has six transmembrane segments (designated S1-S6) and assembles post-translationally to form a tetrameric complex. The Kv2.1 channel consists of four α subunits, in which the core domain of about 300 amino acids in length together assembles to form the main part of the voltage sensing device and the ion-selective pore. The amino and carboxy termini are present in the cytoplasm, so all extracellular domains are located within the core domain. The cytoplasmic N-terminus of the Kv2.1 alpha subunit contains a tetramerization (T1) domain that contains molecular determinants for the specific assembly of the alpha subunit into functional tetrameric channels. The different Kv channel alpha subunits display a high degree of amino acid sequence similarity within the core and T1 domains, but are more distinct in the carboxy and non-T1 amino-terminal domains. These cytoplasmic domains are involved in a wide range of intersubunit interactions in tetramers, as well as in protein-protein interactions that are involved in regulating channel trafficking, localization, and function.
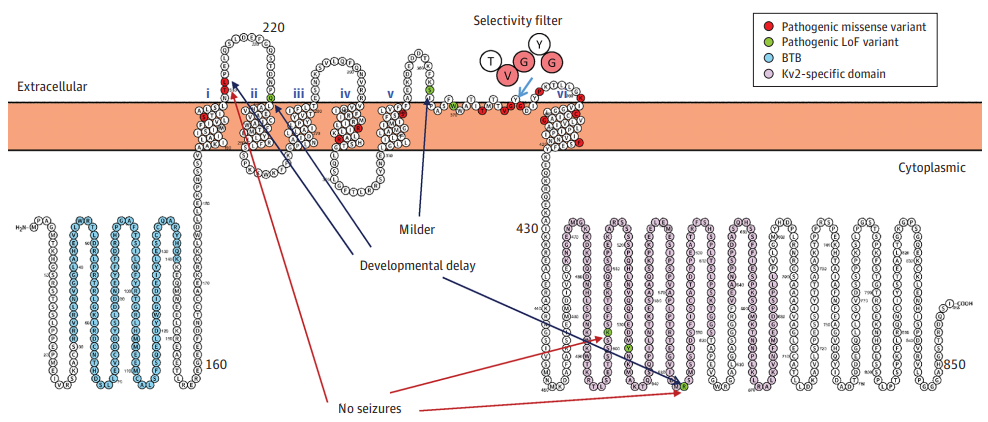
Figure 1. Schematic view of the Kv2.1 (KCNB1) protein in the membrane. (de Kovel CGF, et al.;2017)
KCNB1 Distribution
Kv2.1 voltage-gated K+ channels were found to either diffuse freely at the plasma membrane or be concentrated in micrometer-scale clusters located in the soma, proximal dendrites, and initial segments of hippocampal neuron axons. Therefore, dynamic regulation of Kv2.1 is expected to have an effect on neuronal excitability. Kv2.1 is uniquely localized to large clusters on the soma and the most proximal portion of dendrites in mammalian brain K+ channels. The distribution of Kv2.1 was found in clusters on somatic cells and proximal dendrites. In addition, Kv2.1 was also found proximally in cultured hippocampal neurons.
KCNB1 and Disease
KCNB1 Encephalopathy
More than 45 KCNB1 gene mutations were found to cause KCNB1 encephalopathy. This condition is characterized by abnormal brain function (encephalopathy), recurrent seizures (epilepsy), and developmental delay. Most KCNB1 gene mutations alter a single amino acid, which alters the protein. Other mutations introduce a premature stop signal in the instructions for making the protein, resulting in a shortened, nonfunctional protein. All KCNB1 gene mutations impair Kv2.1 channel function. Channels made with the abnormal protein didn't work properly, causing Kv2.1 channel in the brain to function less. As a result, these channels fail to regulate the flow of potassium ions in neurons, disrupting normal communication between these cells. Impaired channel function disrupts normal brain development, leading to seizures, intellectual disability, and other hallmarks of encephalopathy that occur in this condition.
References
- Bar C, et al.; Expanding the genetic and phenotypic relevance of KCNB1 variants in developmental and epileptic encephalopathies: 27 new patients and overview of the literature. Hum Mutat. 2020, 41(1):69-80.
- de Kovel CGF, et al.; Neurodevelopmental Disorders Caused by De Novo Variants in KCNB1 Genotypes and Phenotypes. JAMA Neurol. 2017, 74(10):1228-1236.
- Misonou H, et al.; Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005, 26(5):743-52.
- Sahoo N, et al.; Oxidative modulation of voltage-gated potassium channels. Antioxid Redox Signal. 2014, 21(6):933-52.
- Torkamani A, et al.; De novo KCNB1 mutations in epileptic encephalopathy. Ann Neurol. 2014, 76(4):529-540.
Inquiry
