- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0038 | Human KCNC2 Stable Cell Line-CHO | KCNC2 | Human | Epithelial-like | INQUIRY |
KCNC2, also known as Kv3.2, is a complete membrane protein that belongs to the Kv3 subfamily of voltage-gated potassium channels. The Kv3 subfamily consists of Kv3.1-3.4 subunits, which can be constructed as homomeric tetramer potassium ion channels or heteromeric tetramer potassium ion channels.
The Kv3.2 channel encoded by the KCNC2 gene contains 6 transmembrane fragments (S1-S6), which are selectively expressed in the central nervous system, especially in the cortex, hippocampus, basal amygdala and GABA in the caudate nucleus can suppress neuronal populations. The channels are characterized by the "quick spike" shooting mode. There are 4 Kv3.2 isoforms (Kv3.2a–Kv3.2d) derived from alternative splicing of the KCNC2 gene, resulting in different cytoplasmic COOH end domains determining their subcellular location. These Kv3.2 specific properties are essential for maintaining effective synaptic transmission in neocortical GABAergic interneurons through continuous high-frequency action potential sequences.
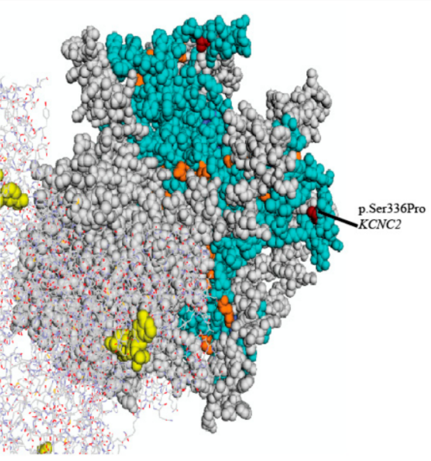
Figure 1. Three-dimensional structural homology model of the voltage-gated potassium channel, Kv3.2 with mapped EA-related mutations. (Neven Maksemous, et al.; 2018)
Studies have found that the characteristics of Kv3.2 channel high-frequency action potentials help to regulate rapid action potential repolarization and the continuous high-frequency discharge of central nervous system neurons. The homogeneous tetramer channel mediates delayed rectification voltage-dependent potassium current, which is activated rapidly at high threshold voltage and slowly inactivated. Potassium ions though the KCNC2 tetramer channels according to its electrochemical gradient. The channel alternates between open and closed conformations in response to the voltage difference across the membrane. The homotetramer and heterotetramer channels formed by KCNC2 contain different proportions of KCNC1, and may also contain other family members; channel properties depend on the type of alpha subunit that is part of the channel. Channel characteristics may be indirectly regulated by nitric oxide (NO) through the association with auxiliary subunits, such as KCNE1, KCNE2, or KCNE3, or through cGMP and pkg-mediated signaling cascades, slowing the activation and inactivation of delayed rectifier potassium channels. In retinal ganglion cells, thalamic cortex and suprachiasmatic nucleus (SCN) neurons, as well as hippocampal and neocortical interneurons, the KCNC2 channel can meet the requirements of high-frequency excitation and continuous very brief action potentials. The sustained maximum action potential firing frequency of inhibitory hippocampal interneurons is negatively regulated by the activation of histamine H2 receptors in a cAMP- and protein kinase (PKA) phosphorylation-dependent manner. By generating action potential (AP) repolarization at nerve endings, the fidelity of synaptic transmission of neocortical GABAergic interneurons is maintained, thereby reducing peak-induced calcium influx and GABA neurotransmitter release. The KCNC2 voltage-gated potassium ion channel requires long-distance synchronization of gamma oscillations in the neocortex. This helps to regulate the circadian rhythm of spontaneous action potential discharges in the suprachiasmatic nucleus (SCN) neurons in a light-dependent manner.
KCNC2 and Disease
In the literature, there is evidence that defects in Kv3.2 channels lead to increased neuronal hyperexcitability by causing repetitive GABAergic interneurons or damage to tonic high-frequency discharges. In particular, studies on mice lacking Kv3.2 channels have shown that cortical inhibition is suppressed and the susceptibility to seizures is increased. Another study showed that the seizure activity of adult gerbils involves changes in the expression of delayed rectifier K+ channels (especially Kv3.1-2) in neurons and astrocytes. Finally, Kv3.2 knockout mice showed defects in high-frequency oscillation modulation, which requires effective regulation of complete GABA to inhibit transmission.
References
- Chandy, K. G., et al.; A family of three mouse potassium channel genes with intronless coding regions. Science. 1990, 247: 973-975.
- LuigiVetri, et al.; A de novo heterozygous mutation in KCNC2 gene implicated in severe developmental and epileptic encephalopathy. European Journal of Medical Genetics. 2020, Volume 63, Issue 4.
- Neven Maksemous, et al.; Whole-Exome Sequencing Implicates SCN2A in Episodic Ataxia, but Multiple Ion Channel Variants May Contribute to Phenotypic Complexity. Int. J. Mol. Sci. 2018, 19(10), 3113.
Inquiry
