- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0138 | Human KCNC4 Stable Cell Line-HEK293 | KCNC4 | Human | Epithelial | INQUIRY |
Potassium channels are the most complex type of voltage-gated ion channels in the body in terms of function and structure. They are present in all eukaryotic cells and have multiple functions, including maintaining membrane potential, regulating cell volume, and regulating the electrical excitability of neurons. The delayed rectification function of potassium channels allows nerve cells to effectively repolarize after an action potential.
KCNC4 Cloning and Expression
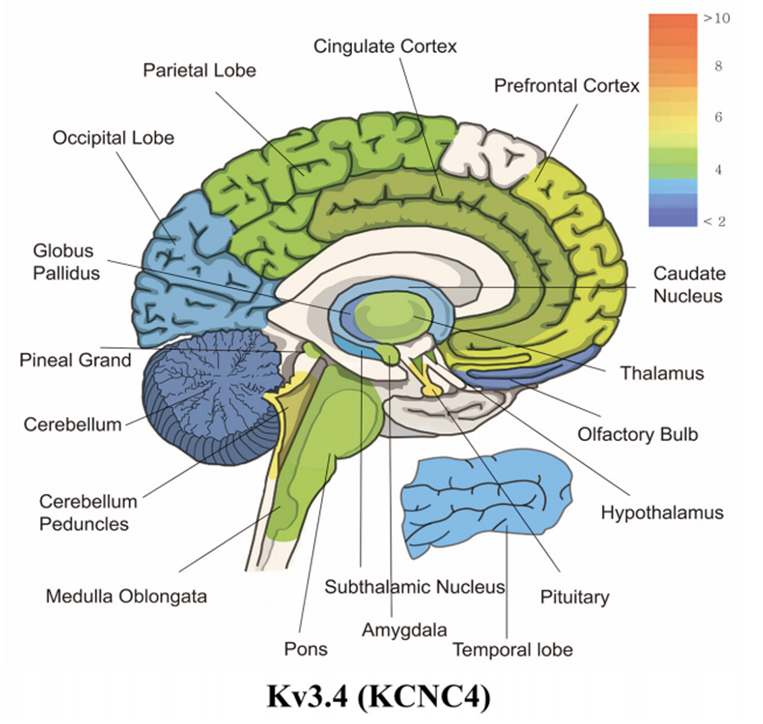
Figure 1. mRNA expression levels of panel kcnc4 (Kv3.4). (Wonjun Noh, et al.; 2019)
Rudy et al. reported in 1991 that the human K+ channel ShIII cDNA (HKShIIIC) was cloned from the brainstem cDNA library. They mapped the KCNC4 gene to 1p21 by in situ hybridization. After that, the scientists will map the KCNC4 gene to chromosome 1p13.3. Further research shows that KCNC4 (Kv3.4) is a complete membrane protein, belonging to the Kv3 subfamily of voltage-gated potassium channels. The Kv3 subfamily consists of Kv3.1-3.4 subunits, which can be constructed as homogeneous potassium tetramer channels or heterogeneous potassium tetramer channels. The KCNC4 protein encoded by the KCNC4 gene has 635 amino acid residues and contains 6 transmembrane fragments (S1-S6). There is a voltage sensor in S4. It is mainly expressed in the brain and kidneys.
KCNC4 Function
KCNC4 is a voltage-gated potassium ion channel, which regulates the transmembrane transport of potassium ions according to the electrochemical gradient of potassium ions. The channel can change its open or closed conformational state according to the voltage difference across the membrane, thereby regulating the potassium ion concentration on both sides of the membrane, thereby affecting the generation of a-type current. In skeletal muscle, voltage-gated potassium channels containing kcnc4 regulate the resting potential of muscle cells.
KCNC4 and Disease
Head and Neck Squamous Cell Carcinoma (HNSCC)
The existence of Kv3.4 potassium channel subunits is important in the development and progression of head and neck squamous cell carcinoma (HNSCC). Researchers of choice have found up-regulated KCNC4 mRNA levels in HNSCC tissue specimens and derived cell lines. Although no significant correlation was found between the positive expression of Kv3.4 in HNSCC and clinical data; however, the expression of Kv3.4 tends to decrease in advanced tumors. Interestingly, patients with Kv3.4-positive abnormal hyperplasia have a significantly higher incidence of laryngeal cancer than patients with negative lesions. In addition, functional studies using HNSCC cells showed that the inhibition of Kv3.4 expression by siRNA resulted in the inhibition of cell proliferation through selective cell cycle arrest in the G2/M phase, without affecting cell apoptosis. Overall, these data prove that Kv3.4 expression often increases during HNSCC tumorigenesis and is significantly associated with a higher cancer risk.
Spinal Cord Injury
Patients with spinal cord injury (SCI) experience chronic pain, which involves central and peripheral nerve mechanisms. Because the dysregulation of the voltage-gated Kv3.4 channel is related to the hyperexcitability of the dorsal root ganglion (DRG) neurons after the sensory nerve is directly injured, the researchers explored its impact on SCI disease. Kv3.4 channels are expressed in DRG neurons, where they help regulate action potential (AP) repolarization in a way that depends on the protein kinase C (PKC)-dependent phosphorylation of the channel inactivation domain to inactivate The adjustment. Two weeks after cervical spine contusion and SCI, the injured rat showed contralateral hypersensitivity to the stimulus, accompanied by repetitive spikes of putative DRG nociceptors. Also in these neurons 1 week after laminectomy and SCI, Kv3.4 channel inactivation was impaired compared with the pure non-surgical control. However, 2-6 weeks after laminectomy, the Kv3.4 channel inactivation returned to its original level. On the contrary, the Kv3.4 current was lowered 2-6 weeks after SCI and remained slowly inactivated. Immunohistochemistry showed that the down-regulation was mainly due to the decreased surface expression of the Kv3.4 channel, because the whole DRG protein and single-cell mRNA transcription levels did not change. In addition, consistent with Kv3.4 channel dysregulation, PKC activation failed to shorten the AP duration of small-diameter DRG neurons. Finally, re-expression of synthetic Kv3.4 currents under dynamic clamping conditions suppressed repetitive spikes in SCI rat neurons.
References
- Ritter D.M., et al.; Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. Journal of Neuroscience the Official Journal of the Society for Neuroscience. 2015, 35(3):1260-73.
- Menéndez S.T., et al.; Expression and clinical significance of the Kv3.4 potassium channel subunit in the development and progression of head and neck squamous cell carcinomas. Journal of Pathology. 2010, 221(4):402-10.
- Chandy, K. G., et al.; A family of three mouse potassium channel genes with intronless coding regions. Science. 1990, 247: 973-975.
- Ghanshani, S., et al.; Genomic organization, nucleotide sequence, and cellular distribution of a Shaw-related potassium channel gene, Kv3.3, and mapping of Kv3.3 and Kv3.4 to human chromosomes 19 and 1. Genomics.1992, 12: 190-196.
- McPherson, J. D., et al.; Chromosomal localization of 7 potassium channel genes. Cytogenet. Cell Genet. 1991, 58: 1979 only.
- Rudy, B., et al.; Cloning of a human cDNA expressing a high voltage-activating, TEA-sensitive, type-A K+ channel which maps to chromosome 1 band p21. J. Neurosci. Res. 1991, 29: 401-412.
- Wonjun Noh, et al.; Transient Potassium Channels: Therapeutic Targets for Brain Disorders. Frontiers in Cellular Neuroscience. 2019, 13: 265.
Inquiry
