- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0139 | Human KCND2/KCNIP2 Stable Cell Line-HEK293 | KCNIP2 | Human | Epithelial | INQUIRY |
| ACC-RI0142 | Human KCND3/KCNIP2 Stable Cell Line-CHO | KCNIP2 | Human | Epithelial-like | INQUIRY |
KCNIP2 gene is a member of the family of voltage-gated potassium (Kv) channel interacting proteins (KCNIPs). Studies have shown that members of the KCNIP family are small calcium binding proteins. They all have similar EF hand-shaped domains and are different from each other at the N-terminus. Structural analysis shows that the KCNIP family is part of the native Kv4 channel complex. They can regulate type A currents, thereby regulating neuronal excitability in response to changes in intracellular calcium. Multiple alternatively spliced-transcript variants encoding different isotypes have been identified from this gene.
KCNIP2 Cloning and Expression
In the brain and heart, rapidly inactivating (Type A) voltage-gated potassium (Kv) currents run at subthreshold membrane potentials to control the excitability of neurons and cardiomyocytes. Scientists discovered the Kv3 channel interacting protein, KCHIP, which binds to the cytoplasmic amino terminus of the Kv4-α subunit. Further research found that KCHIP2 cDNA encodes a 252 amino acid protein, which has a different N terminal but has 70% amino acid similarity with KCHIP1 and KCHIP3. The entire carboxy terminal 185 amino acid core domain contains 4 EF hand structures. . Through gene mapping analysis, it is found that hKCNIP2 is located at 10q24.
KCNIP2 Gene Function
The researchers found that the circadian rhythm is related to the susceptibility of mice to ventricular arrhythmia. Specifically, they showed that cardiac ion channel expression and QT interval duration (an indicator of myocardial repolarization) exhibit endogenous circadian rhythms under the control of the clock-dependent oscillator Kruppel-like factor 15 (KLF15). Klf15 transcription controls the rhythmic expression of KChIP2, which is a key subunit required to generate transient outward potassium currents. Lack or overdose of Klf15 can lead to loss of rhythmic QT variants, abnormal repolarization, and increased susceptibility to ventricular arrhythmias.
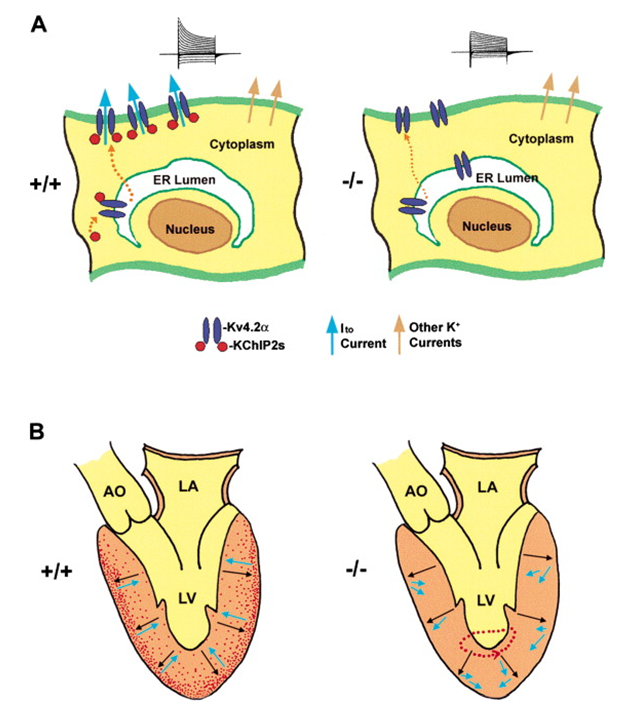
Figure 1. A Schematic Model of How a Deficiency in KChIP2 May Abolish Ito Expression in Cardiac Myocytes and Confer Susceptibility to Ventricular Arrhythmias. (Hai-Chien Kuo, et al.; 2001)
Kv4.3 channels conduct transient outward K+ currents in the human heart and brain, where they mediate the early stages of action potential repolarization. KChIP2 protein is a member of a new type of calcium sensor that can regulate the surface expression and biophysical properties of Kv4 K+ channels. Among them, KChIP2e and KChIP2f are produced by C-terminal alternative splicing, and KChIP2g can be produced by N-terminal alternative splicing. KChIP2e and KChIP2f are expressed in the human atrium, while KChIP2g is mainly expressed in the brain. The functional study was carried out by co-expressing the KChIP2 subtype with the Kv4.3 channel in Xenopus oocytes. It was found that KChIP2e caused a decrease in current amplitude, accelerated inactivation and slower recovery of Kv4.3 current inactivation; KChIP2f increased the current amplitude and slowed down the inactivation speed, but did not change the inactivation recovery or the half of the Kv4.3 channel maximum Inactivation voltage; KChIP2g increases the current amplitude, slows down the inactivation rate, and shifts half of the maximum inactivation voltage to a more negative potential. The biophysical changes caused by these alternatively spliced KChIP2 proteins are significantly different from the previously described KChIP2 proteins, and are expected to increase the diversity of natural transient outward K+ currents.
KCNIP2 and Disease
Existing studies have shown that the transient outward K(+) current (I(to1)) irrelevant to Ca(2+) in the heart is responsible for the initial phase of repolarization. Among them, the α subunit of the hKv4.3 K(+) channel contributes to the I(to1) current in many regions of the human heart. In heart failure and atrial fibrillation, the down-regulation of the hKv4.3 transcript leads to a decrease in I(to1) conductance. The KChIP family of calcium sensors can regulate the a-type potassium channels of the Kv4 K(+) channel subfamily. Therefore, hKChIP2 is the true β subunit that regulates human heart I(to1). Its mutations may be involved in a variety of heart diseases.
References
- An, W. F., et al.; Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000, 403: 553-556.
- Jeyaraj, D., et al.; Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012, 483: 96-99.
- Kuo, H.-C., et al.; A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I-to, and confers susceptibility to ventricular tachycardia. Cell . 2001, 107: 801-813.
- Decher N, et al.; hKChIP2 is a functional modifier of hKv4.3 potassium channels: cloning and expression of a short hKChIP2 splice variant. Cardiovasc Res. 2001, 52(2):255-64.
- Hai-Chien Kuo, et al.; A Defect in the Kv Channel-Interacting Protein 2 (KChIP2) Gene Leads to a Complete Loss of Ito and Confers Susceptibility to Ventricular Tachycardia. Cell. 2001, 107(6): P801-813.
Inquiry
