- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0114 | Human KCNJ1 Stable Cell Line-HEK293 | KCNJ1 | Human | Epithelial | INQUIRY |
KCNJ1 is a gene encoding inwardly rectified potassium channels protein components. Among them, inward rectifier potassium channels usually control the resting potential and membrane excitability.
The potassium channel encoded by the KCNJ1 gene plays a key role in the transport of positively charged potassium ions in and out of the cell, as well as the cell's ability to generate and transmit electrical signals. Existing studies have shown that the specific function of potassium channels depends on its protein composition and its location in the body. The channel formed by the KCNJ1 protein, also called ROMK, is mainly found in the kidney. ROMK is one of several proteins that work together to regulate the flow of ions into and out of kidney cells. In particular, the transport of potassium ions through ROMK is necessary for the normal function of another ion transporter, NKCC2 (produced by the SLC12A1 gene). This transporter plays an important role in the reabsorption of salt (sodium chloride) from the urine into the blood. The correct retention of salt affects the body's fluid levels and helps maintain blood pressure.
KCNJ1 Cloning and Expression
In 1993, Ho et al. cloned rat Romk1 cDNA from the kidney, and the results showed that the predicted protein had only 2 transmembrane domains, while there were 6 transmembrane domains in other potassium channels. In 1994, Yano et al. used primers based on the rat Romk1 sequence to perform PCR to create probes from a human kidney cDNA library. Then the library was screened to isolate two types of cDNA. The two isomers of KCNJ1 are called ROMK1A and ROMK1B, and their 5'ends are different due to alternative splicing. Both subtypes of KCNJ1 are detected in the kidney, but other tissues only show type A. Among them, ROMK1A has 93% similarity with the rat homologue. In the same year, the researchers used rat kidney Romk1 potassium channel cDNA to clone homologues from human kidneys. In addition to the human homologs of rat genes, four other transcripts formed by alternative splicing of a single human gene were also characterized. All five transcripts have a common 3'-exon, which is involved in encoding most of the channel proteins. In the three subtypes, translation begins with the start codon contained in the exon.
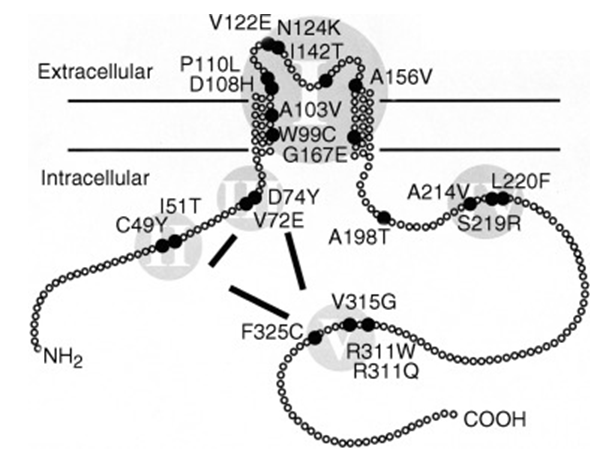
Figure 1. Distribution and clustering of missense mutations in the ROMK channel molecule.(Nikola Jeck, et al.; 2001)
KCNJ1 and Disease
Bartter Syndrome
Dozens of KCNJ1 gene mutations have been found in patients with type II Bartter syndrome. This form of disorder can cause serious or life-threatening health problems, which manifest before or shortly after birth. Some KCNJ1 gene mutations that cause Bartter syndrome change a single amino acid in the ROMK protein. These mutations prevent the protein from reaching the cell membrane or alter the channel's ability to transport potassium ions. Other mutations in the KCNJ1 gene can delete amino acids in the protein or cause an abnormally short, non-functional version of ROMK. The lack of functional ROMK affects the normal activity of the NKCC2 protein, preventing it from delivering ions to kidney cells. Therefore, the kidneys cannot reabsorb salt normally, and the excess salt will be lost through the urine (salt consumption). The abnormal loss of salt disrupts the normal balance of sodium, potassium and other ions in the body. These imbalances constitute the main characteristics of Bart's syndrome.
Other Disorders
Studies have shown that normal mutations (polymorphisms) of the KCNJ1 gene may help explain differences in blood pressure in different populations. Certain rare polymorphisms seem to prevent high blood pressure, and researchers speculate that other genetic variants may increase the risk of hypertension. Changes in the KCNJ1 gene may affect blood pressure by changing the kidney's ability to reabsorb salt into the blood.
References
- Ho, K., et al.; Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature.1993, 362: 31-38.
- Yano, H., et al.; Alternative splicing of human inwardly rectifying K+ channel ROMK1 mRNA. Molec. Pharm. 1994, 45: 854-860.
- Krishnan, S. N., et al.; Isolation and chromosomal localization of a human ATP-regulated potassium channel. Hum. Genet. 1995, 96: 155-160.
- Lopes, C. M. B., et al.; Alterations in conserved Kir channel-PIP(2) interactions underlie channelopathies. Neuron. 2002, 34: 933-944.
- Derst C, et al.; Mutations in the ROMK gene in antenatal Bartter syndrome are associated with impaired K+ channel function. Biochem Biophys Res Commun. 1997, 230(3):641-5.
- Jeck N, et al.; Functional heterogeneity of ROMK mutations linked to hyperprostaglandin E syndrome. Kidney Int. 2001, 59(5):1803-11.
- Ji W, et al.; Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008, 40(5):592-599.
- Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol. 2009, 297(4):F849-63.
- Nikola Jeck, et al.; Functional heterogeneity of ROMK mutations linked to hyperprostaglandin E syndrome. Kidney International. 2001, 59(5):1803-1811.
Inquiry
