- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0105 | Human KCNMA1 Stable Cell Line-CHO | KCNMA1 | Human | Epithelial-like | INQUIRY |
The K+ channel that can be activated by a large conductance voltage and Ca2+ is also called a BK channel. Unlike other potassium channels, it can be activated by Ca2+ ions in the cell or depolarized by the membrane. The study found that the BK channel consists of 4 alpha subunits and 4 optional auxiliary beta subunits. Among them, the α subunit that forms the pore is encoded by the KCNMA1 gene, which generates multiple subtypes through alternative splicing. The 4 beta subunits are encoded by different genes and show tissue-specific expression.
KCNMA1 Cloning and Expression
KCNMA1 was first discovered in the study of mutations in Drosophila. This gene can specifically eliminate fast calcium-activated potassium currents in Drosophila adult and larval muscles and larval neurons. Later, Pallanck and Ganetzky cloned human and mouse homologues of Drosophila genes, and proved that the encoded polypeptide has more than 50% amino acid similarity with Drosophila homologues. Tseng-Crank et al. used probes based on Drosophila and mouse Slo sequences to screen human brain cDNA libraries and obtained clones representing nine human Slo splice variants. All isomers have 22 serines near the N-terminus, followed by 10 hypothetical hydrophobic fragments, of which the first 6 are expected to span the membrane and surround the pore. All amino acid insertions caused by alternative splicing occurred in a large cytoplasmic c-terminal domain between hydrophobic fragments 8 and 10, and most of the insertions introduced phosphorylation sites. Northern blot detected that the variable expression levels of SLO transcripts in all tissues were 10.2, 6.7, 4.7, and 4.1 kb, respectively, with the highest expression levels in the brain, aorta and skeletal muscle. The 6.7 kb transcript is expressed differently in all specific brain regions, with the highest expression in the amygdala, caudate nucleus, cerebral cortex, hippocampus, hypothalamus and spinal cord. The 10.2 kb transcript was also detected in most of the brain regions tested.
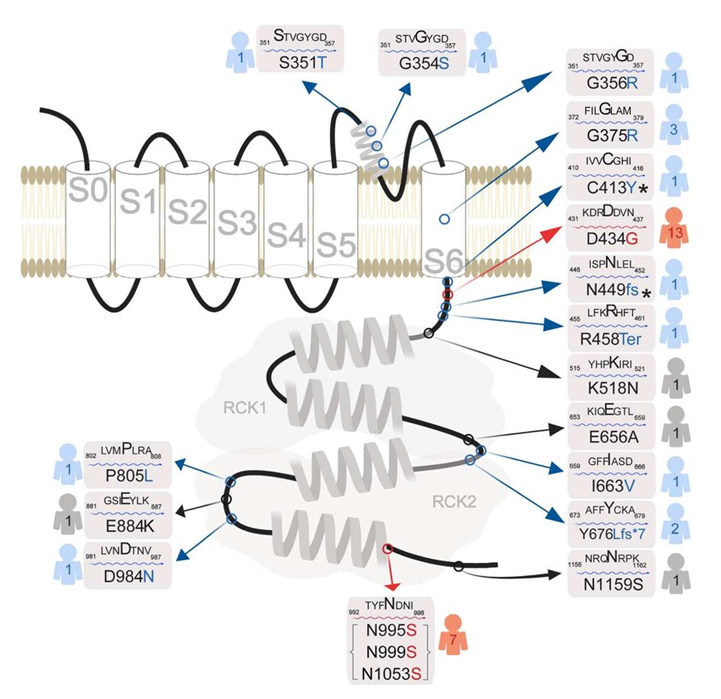
Figure 1. Human KCNMA1 mutations. (Bailey, C. S., et al.; 2019)
KCNMA and Disease
Clinical studies have found that mutations in the KCNMA1 gene mainly lead to a series of major neurological diseases.
Paroxysmal Non-motor Dyskinesia 3
The researchers identified heterozygous missense mutations in all 13 affected members of the paroxysmal non-motor-induced dyskinesia 3 family with or without generalized epilepsy. The results show that the BK channel of the D434G mutant has a significantly larger macroscopic current. Single-channel recordings showed that the Ca2+ sensitivity increased by a factor of 3 to 5, leading to an increase in the possibility of opening the channel. Researchers believe that the enhancement of BK channels in the body can increase excitability by inducing rapid repolarization of action potentials, and cause generalized epilepsy and paroxysmal dyskinesia by firing neurons at a faster rate. Studies have shown that BK channel blockers can be used in the treatment of epilepsy and paroxysmal movement disorders.
Cerebellar Atrophy
In patients with cerebellar atrophy, homozygous mutations in the KCNMA1 gene were identified. The mutation was discovered by a combination of homozygous mapping and exome sequencing, and confirmed by Sanger sequencing, and was isolated from the disease in the family. The functional study of the variant and the study of patient cells have not been conducted, but it is expected that the variant will result in a complete loss of channel function.
Susceptibility to Idiopathic Generalized Epilepsy 16
The researchers identified de novo heterozygous missense mutations in 16 patients with 2 unrelated idiopathic generalized epilepsy. In vitro functional expression studies in HEK293 cells showed that, compared with wild-type, mutant channels are easier to open at a given calcium level than wild-type, perform ion flow faster and longer, and generate larger currents, Which is consistent with the function gain effect.
References
- Ahluwalia, J., et al.; The large-conductance Ca(2+)-activated K+ channel is essential for innate immunity. Nature. 2004, 427: 853-858.
- Alioua, A., et al.; Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J. Biol. Chem. 2008, 283: 4808-4817.
- Bailey, C. S., et al.; KCNMA1-linked channelopathy. J. Gen. Physiol. 2019, 151: 1173-1189.
- Chen, L., et al.; Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J. Biol. Chem. 2005, 280: 33599-33609.
- Du, W., et al.; Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nature Genet. 2005, 37: 733-738.
- Higgins, J. J., et al.; Dysregulation of large-conductance Ca(2+)-activated K(+) channel expression in nonsyndromal mental retardation due to a cereblon p.R419X mutation. Neurogenetics. 2008, 9: 219-223.
Inquiry
