- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0180 | Human P2RX1 Stable Cell Line-HEK293 | P2RX1 | Human | Epithelial | INQUIRY |
P2 receptors are widely distributed in various tissues and systems throughout the body and are divided into P2X and P2Y receptors according to their molecular structures and signal transduction mechanisms. It is now generally believed that P2X is a ligand-gated cation channel, and P2Y is a G-protein coupled receptor. P2X purinoceptor 1 is a ligand-gated cation channel protein encoded by the P2RX1 gene in humans.
The amino acid sequences of the seven subunits of P2X receptors have at least 50% homology. The number of amino acids varies from 379 to 595. According to the amino acid sequence of the P2X receptor subunit, the structure of the receptor is deduced to include two transmembrane domains of M1 and M2, an extracellular loop connecting M1 and M2, and intracellular C-terminal and N-terminal of different lengths. The N-terminus is shorter (<30 amino acids), and the C-terminus varies in length from 25 residues to 240 residues, and its intracellular phosphorylation site is located at the C-terminus. The binding site of ATP molecule and receptor is still unclear. Some data report that the Lys residue in the extracellular loop plays a role in nucleotide binding, and some data indicate that the P2X receptor may have two ligand binding sites. Like other ion channels, P2X receptors are oligomeric protein bodies composed of multiple subunits, but the number of subunits required to constitute the receptor is still unclear. Studies have shown that its trimer may be the smallest functional unit.
P2RX1 Clone
Valera et al. (1994) cloned the rat P2X1 gene. The researchers then isolated cDNA encoding human P2X1 or P2RX1 by screening the bladder library with rat cDNA. The predicted 399 amino acid protein sequence of human P2RX1 is 89% homologous to rat and mouse sequences. Northern blot results showed that P2X1 was mainly expressed in 2.6 ~ kb mRNA in various tissues. 1.8-, 3.6- and 4.2 kb mRNAs were also detected in some tissues. Sun et al. (1998) isolated platelet cDNA corresponding to the 1.8-kb P2RX1 transcript. Although the coding regions are identical, the platelet transcript has a longer 5' untranslated region and a truncated 3' untranslated region. The researchers believe that the different transcripts are caused by alternative promoters or splicing. In Western blot experiments of extracts from mammalian cells expressing P2X1, the apparent molecular mass of the protein was 70kD, which was significantly higher than the predicted amino acid sequence of 45kD. The researchers pointed out that this difference may be due to the glycosylation of the extracellular domain.
Gene Function of P2RX1
Through heterologous expression of human P2X1 in Xenopus oocytes, scientists discovered that the receptor is sensitive to the purinergic agonists ATP and α,β-methylene ATP. Further investigation revealed that the P2X1 receptor exhibited ATP- and ADP-stimulated calcium influx when expressed in astrocytoma cells.
Electrophysiological and biochemical studies have shown that the P2X1 receptor is expressed in human and rat platelets, rat basophilic leukemia cells, and phorbol myristate acetate-differentiated bone marrow cells. Although these findings suggest that P2X1 receptors are present in blood leukocytes and platelets, Clifford et al. (1998) found that P2X1 receptors are significantly expressed in human platelets, but not in mature neutrophils, monocytes or blood lymphocytes. Studies of nucleotide-induced changes in Ca(2+) influx/mobilization suggest that platelet P2X1 receptors are pharmacologically distinct from the well-characterized P2Y1 receptors. ATP is the most potent physiological nucleotide agonist of the P2X1 receptor, while ADP is a functionally equivalent but less potent agonist. In contrast, the P2Y1 receptor displayed absolute selectivity for ADP as a physiological agonist and was antagonized by high concentrations of extracellular ATP. These different selectivities suggest that platelets may use ATP and ADP for different types of regulation, and suggest that the P2X1 receptor has a unique role in hemostasis or thrombosis.
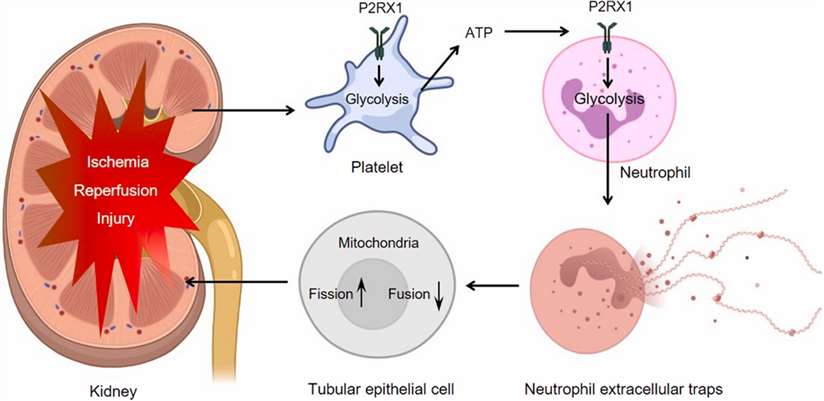
Figure 1. Targeting P2RX1 alleviates renal ischemia/reperfusion injury by preserving mitochondrial dynamics. (Shaoyong Zhuang; et al.; 2021)
Further investigation revealed that the use of α, β-methylene ATP elicited a fast transient P2X1 receptor-mediated increase in Ca2+, whereas ADP elicited a slower but higher and longer P2Y receptor response. The Ca2+ response to α, β-methylene ATP and ADP is accelerated and amplified, suggesting that ionotropic P2X1 plays an initiating role in the subsequent activation of metabotropic P2Y receptors during platelet stimulation.
References
- Gever JR, et al.; Pham acology of P2x channels. Pflugers Arch. 2006, 452(5): 513-537.
- Valera, S., et al.; A new class of ligand-gated ion channel defined by P-2X receptor for extracellular ATP. Nature. 371: 516-519, 1994.
- Sun, B., et al.; P2X1 purinoceptor in human platelets: molecular cloning and functional characterization after heterologous expression. J. Biol. Chem. 273: 11544-11547, 1998.
- Clifford, E. E., et al.; The P2X(1) receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood.91: 3172-3181, 1998.
- Vial, C., et al.; A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Brit. J. Pharm. 135: 363-372, 2002.
- Shaoyong Zhuang; et al.; Targeting P2RX1 alleviates renal ischemia/reperfusion injury by preserving mitochondrial dynamics. Pharmacological Research. 2021, Volume 170, 105712.
Inquiry
