- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- P2RX2
P2RX2
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0182 | Human P2RX2 Stable Cell Line-HEK293 | P2RX2 | Human | Epithelial | INQUIRY |
P2RX2 is the gene encoding the P2X2 receptor, which aggregates in the form of trimers to form ligand-gated ion channels that are gated by extracellular ATP. P2X2 receptors mediate a variety of cellular responses, including excitatory postsynaptic responses in sensory neurons.
P2RX2 Cloning and Expression
The rat P2X2 gene was cloned by Brake et al. (1994). The researchers then cloned rat genes from the dorsal root ganglia and demonstrated that P2X3 was co-expressed with P2X2. Their combination produced ATP-activated currents similar to those seen in sensory neurons. In conclusion, sensory neurons of ATP-gated channels are formed by heteromerization of specific P2X receptor subunits. In 1999, Lynch et al. cloned and characterized the human P2X2 gene. The full-length cDNA encodes a predicted protein of 471 amino acids, which is 68% identical to that of rat P2X2. The predominant mRNA in pancreas was detected by Northern blot at approximately 2.4 kb; in addition, faint bands of the same size were detected in heart and brain. Two of the four isolated P2X2 cDNAs expressed in Xenopus oocytes expressed functional ion channels.
Through RT-PCR analysis, the researchers found that two P2X2 splice variants were expressed in the guinea pig cochlea. Variant-1 is expressed in the sensory epithelium of the organ of Corti, while variant-2, which encodes the desensitized isoform, is expressed in primary auditory neurons. Immunohistochemical analysis detected the highest expression of P2X2 in stereocilia of endolymph-facing hair cells, in deits cells in perilymphatic compartments, and in afferent neurons of the spiral ganglia. Immunoelectron microscopy localizes P2X2 to postsynaptic junctions of inner and outer hair cells. Thus, disorders associated with P2RX2 include deafness, autosomal dominant, and autosomal dominant nonsyndromic sensorineural hearing loss types.
P2RX2 Function
Transmission-gated cation channels are detectors of excitatory chemical signals at synapses in the nervous system. The researchers showed that the structurally distinct alpha-3-beta-4 nicotinic and P2X(2) channels influence each other when co-activated. Activation of one channel type affects the different kinetic and conductance states of the other channel, and co-activation leads to non-additive responses due to inhibition of both channel types. The state-dependent inhibitory effect of mutant P2X(2) channel on nicotinic channels was the most obvious, and the inhibitory effect was weakened when the channel expression density was low. In synaptically coupled myoenteric neurons, nicotine-fast excitatory postsynaptic currents are blocked during activation of endogenously coexpressed P2X channels. Later, it was also reported that ATP is a key neurotransmitter connecting taste buds and sensory nerve fibers. Inheritance of ionotropic purinergic receptors (P2X2 and P2X3) abolishes taste responses in taste nerves, although these nerves still respond to touch, temperature and menthol. Likewise, P2x knockout mice had greatly reduced behavioral responses to sweeteners, glutamate, and bitter substances. Furthermore, using isolated guinea pig outer hair cells (OHCs), the scientists found that ATP-activated P2RX2 affects OHC electrokinetic energy, a stimulus-induced change in hair cell length. These acts as an amplifier to determine hearing sensitivity and frequency selectivity. It is found that ATP reduces the OHC EMF-related nonlinear capacitance and shifts its voltage dependence toward depolarization. Drugs that block P2RX2 activation or remove extracellular but not intracellular Ca(2+) abolish the ATP effect.
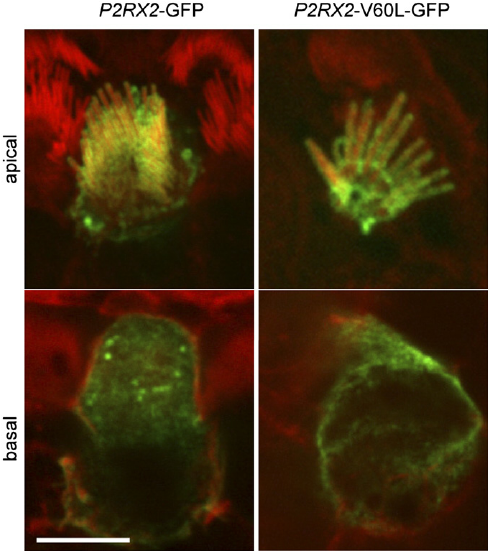
Figure 1. Localization of P2X2 receptors in hair cells. (Denise Yan, et al.; 2013)
In neonatal rat tissue, we found that P2RX2 localized to the apical plasma membrane of hair cells in the organ of Corti, including solid-phase ciliary bundles. Expression patterns of intracellular P2X2 receptors suggest that P2X2 receptors are synthesized and compartmentalized in the endoplasmic reticulum and Golgi complex. This distribution is consistent with the localization of P2RX2 on the lymphatic surface within sensory hair cells and other epithelial cells of the cochlear septum.
Studies in the field of hearing have found that efferent feedback to the cochlea rapidly adapts to intense sounds, starts well below the safe upper limit of hearing, and induces a temporary threshold shift to protect the cochlea from subsequent overstimulation. Both intracochlear potential and hair cell membrane potential are reduced by activation of purinergic signaling. By comparing auditory brainstem responses to increased noise duration and intensity in wild-type and P2RX2 knockout mice, the scientists found that P2RX2 channels are necessary for the occurrence of temporal threshold shifts. Activation of P2RX2 channels by increasing sound intensity reduces hair cell sound transduction and synaptic transmission.
References
- Brake, A. J., et al.; New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994, 371: 519-523.
- Lynch, K. J., et al.; Molecular and functional characterization of human P2X(2) receptors. Molec. Pharm. 1999, 56: 1171-1181.
- Khakh, B. S., et al.; State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000, 406: 405-410.
- Finger, T. E., et al.; ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005, 310: 1495-1499.
- Yu, N., et al.; ATP activates P2x receptors and requires extracellular Ca(2+) participation to modify outer hair cell nonlinear capacitance. Pflugers Arch. 2008, 457: 453-461.
- Yan, D., et al.; Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Nat. Acad. Sci. 2013, 110: 2228-2233.
- Denise Yan, et al.; Mutation of the ATP-gated P2X2 receptor leads to progressive hearing loss and increased susceptibility to noise. Proceedings of the National Academy of Sciences. 2013, 10(6)
Inquiry
