- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- P2RX3
P2RX3
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0184 | Human P2RX2/P2RX3 Stable Cell Line-HEK293 | P2RX3 | Human | Epithelial | INQUIRY |
| ACC-RI0186 | Human P2RX3 Stable Cell Line-HEK293 | P2RX3 | Human | Epithelial | INQUIRY |
In 1972, Burnstock first proposed the concept of purinoceptor and officially named it in 1978. Purinoceptors are divided into P1 and P2 receptors. P2 receptors can bind to the neurotransmitter ATP and undergo a series of conformational changes, and have an important function of transmitting information inside and outside cells. P2 receptors are divided into two categories, including ionotropic P2X receptors and metabolic P2Y receptors. At present, there are 7 subtypes of P2X receptors (P2X1-P2X7) cloned in mammals, all of them are non-selective cation channels, which can penetrate Na+, K+, Ca2+ and other cations. P2X3 receptors are widely distributed in the body and are involved in a variety of physiological and pathological responses, including bladder urination, chronic pain, hypertension, and cough. P2X3 receptors are expressed in bladder afferent nerve fibers and play an important role in bladder voiding function. In the absence of P2X3 receptors, the frequency of urination in mice was also reduced. In addition, P2X3 receptors are also involved in the process of chronic pain. After P2X3 receptor knockout, the mechanical allodynia in mice can be significantly relieved. In addition, P2X3 receptors are also expressed in carotid arteries and may serve as potential targets for the control of hypertension in humans. Inhibition of P2X3 receptors can improve the reflex function of spontaneous cardiac baroreceptors and help inhibit sympathetic nerves in rats, thereby exerting an antihypertensive effect. The P2X3 receptor is also expressed in vagal nerve fibers of the respiratory system, and upon activation by its agonist ATP, may enhance the response of guinea pigs to cough stimuli. The use of AF-219, a P2X3 receptor inhibitor, can significantly improve cough frequency and severity in patients with chronic cough. It can be seen that the P2X3 receptor is an emerging drug target, and the research on its regulation mechanism is of great significance.
Structure of the P2X3 Receptor
The structure and function of P2X receptors show certain subtype and species differences, so it is very necessary to analyze the structure of different P2X receptors. So far, different P2X receptor structures have been resolved by X-ray crystallography: the first is the apo state structure of zebrafish P2X4 (zfP2X4), and the second is its ATP-bound open state; subsequently, the crystal structures of human P2X3 (hP2X3), amblyommamaculatum P2X (AmP2X), panda P2X7 (PdP2X7) and chicken P2X7 (ckP2X7) in different states were solved successively. Although there are differences in subtypes and species of P2X receptors, there is no obvious difference in the basic structure of P2X receptors, and they are all trimers composed of three homologous or heterologous subunits. Each subunit is shaped like a jumping dolphin. Taking the hP2X3 receptor as an example, the subunits can be divided into head, upper body, right flipper (RF), left flipper (LF), dorsal fin (DF), and lower body (LB) according to the shape of dolphins. The extracellular domain is a hydrophilic domain whose transmembrane domain (TM) consists of 6 alpha-helices and an intracellular terminus. The desensitized and open hP2X3 receptors have similar extracellular domains and binding pockets, but there are significant differences in the gate and transmembrane domains. While the open state of the hP2X3 receptor is partially different from the open state of the earlier resolved zfP2X4 receptor, it also has an intracellular motif known as the "cytoplasmic cap". The apo structure of hP2X3 is also quite different from that of zfP2X4, and its transmembrane domain is longer and more complete.
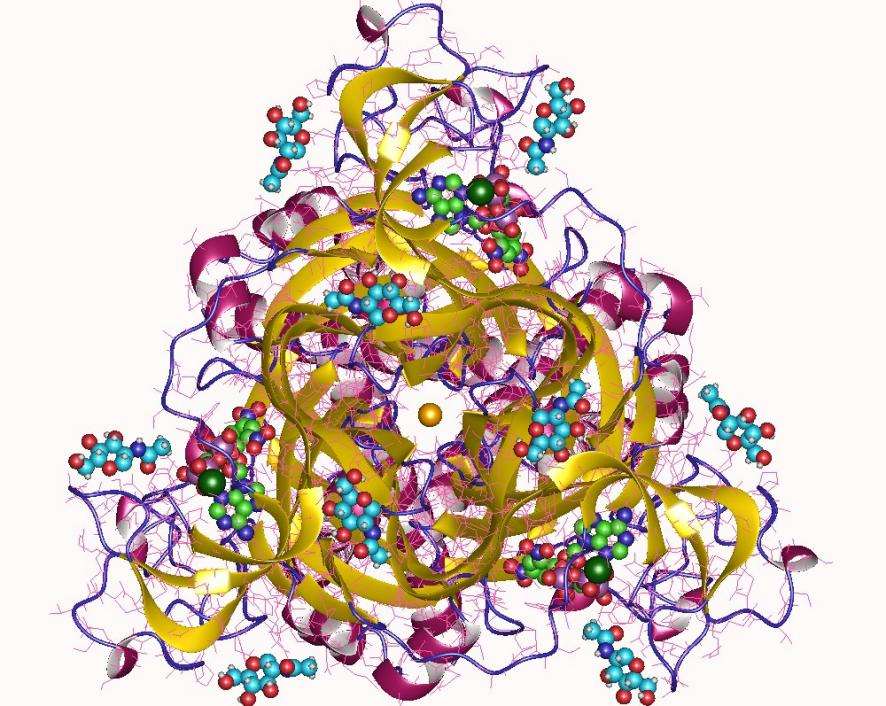
Figure 1. The structure of P2RX3. (From wikipedia.org)
Activators and Activation Mechanisms of P2X3 Receptors
Activators of P2X3 receptors are generally ATP and its derivatives or analogs. ATP, the most common activator of the P2X3 receptor, binds at the interface formed by the interaction of adjacent subunits. The ATP-binding pocket consists of the head and left fin of one subunit and the dorsal and body parts of an adjacent subunit. Also known as the "binding jaw", the closure of this pocket plays an important role in the activation of P2X3 receptors. Free ATP (ATP4−), the most active form of ATP, activates all P2X receptors including P2X3 receptors. Under the action of ATP4−, the activation and desensitization rates of different P2X receptors are significantly different, among which P2X3 receptors are rapidly activated and desensitized. The activation mechanism of P2X3 receptors is similar to that of P2X4 receptors. ATP interacts with amino acids in the binding pocket, causing the head and left fin regions of P2X receptors to move downward, and the dorsal fin region upward, driving the lower body region and transmembrane region expand outward, causing the channel to open. Among them, the "cytoplasmic cap" structure of hP2X3 can promote the opening of channel pores, thereby stabilizing the open state. In addition, MgATP2- is also an activator of the P2X3 receptor and may have higher affinity compared to ATP4−. Although Mg2+ can inhibit the function of P2X receptors, MgATP2- formed after Mg2+ binds to ATP can effectively activate P2X3 and P2X1 receptors. In the presence of 5 mmol/L MgCl2, more than 90% of P2X3 and P2X1 receptors were observed to be activated by MgATP2-, while only about 10% of P2X4 receptors were activated. At present, the activation mechanism of MgATP2- is not clear.
P2X3 Receptor Inhibitors and Their Mechanism of Action
P2X3 receptors are abundantly expressed in primary afferent neurons and play an important role in chronic cough, bladder sensory function, pain perception, and taste transmission. Most of the drugs targeting P2X3 receptors are their inhibitors, and by inhibiting their functions, they can achieve the purpose of antitussive, reducing overactive bladder, and lowering blood pressure. P2X3 receptor inhibitors are divided into competitive inhibitors and non-competitive inhibitors. Non-competitive inhibitors are often called allosteric modulators. Compared with traditional competitive inhibitors, allosteric inhibitors have a wider range of Select the side, you can reduce the side effects of the drug.
References
- Burnstock G. Purinergic nerves. Pharmacol Rev.1972, 24:509-81.
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001, 2:165-74.
- Gao XF, et al.; Pirt reduces bladder overactivity by inhibiting purinergic receptor P2X3. Nat Commun. 2015, 6: 7650.
- Coddou C, et al.; Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011, 63: 641-83.
- Kawate T, et al.; Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009, 460: 592-8.
- Mansoor SE, et al.; X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature. 2016, 538: 66-71.
Inquiry
