- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0188 | Human P2RX4 Stable Cell Line-HEK293 | P2RX4 | Human | Epithelial | INQUIRY |
P2X purinoceptor 4 (P2RX4) is a membrane channel protein encoded by the human P2RX4 gene, located on human chromosome 12q24.31. P2RX4 is a member of the ATP purinoceptor family, also known as the ATP-gated P2X receptor cation channel family. All P2X receptors, including P2RX4, can be activated by ATP, allowing Ca influx into cells, causing changes in cellular homeostasis. Studies have found that P2RX2 is widely expressed in different tissues and cell types, including germline cells, airway cells, microglia and cardiomyocytes as well as neutrophils, eosinophils, mast cells, T and B lymphocytes.
P2RX4 Structure
The P2X receptor structure consists of three subunits that form an extended trimer with three ATP binding sites. Subunits of P2X receptors can associate to form homo- or hetero-trimers that differ in their electrophysiological and/or pharmacological properties. Each subunit has an intracellular N- and C-terminus linked by two transmembrane helices (TM1 and TM2) to a large ectodomain that forms an ATP-binding pocket with another P2X ectodomain. Determination of crystal structures of N- and C-terminally truncated zebrafish P2X4 receptors without ATP or with ATP binding revealed the original new fold for each subunit. Binding of extracellular ATP to P2X receptors induces receptor opening, allowing Ca2+ and Na+ entry and K+ efflux.
P2RX4 and Disease
P2RX4 and Pain
Brain-derived neurotrophic factor (BDNF) is expressed in neurons and immune cells. It has multiple roles, including a neurotrophic factor during development and a role in adult learning. BDNF is also implicated in neuropathic pain: a series of studies have shown that de novo P2RX4 expression triggers the release of BDNF from activated spinal microglia, which are the resident macrophages of the central nervous system. Binding of BDNF to neuronal TrkB receptors downregulates the cotransporter KCC2, resulting in a shift in GABA transmission from inhibitory to excitatory, followed by allodynia and mechanical hypersensitivity. Thus, P2RX4 is a mysterious but intriguing candidate for the treatment of pathological pain potential therapeutic targets.
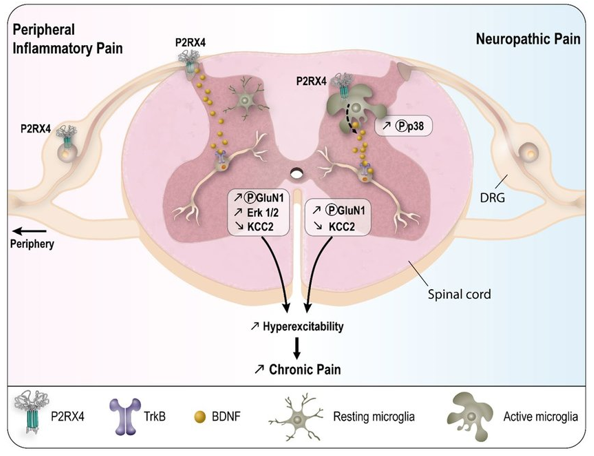
Figure 1. Summary of P2RX4 roles in long lasting inflammation and neuropathic pain. (Sarah Lalisse, et al.; 2018)
P2X purinoceptor 4 (P2RX4) is a membrane channel protein encoded by the human P2RX4 gene, located on human chromosome 12q24.31. P2RX4 is a member of the ATP purinoceptor family, also known as the ATP-gated P2X receptor cation channel family. All P2X receptors, including P2RX4, can be activated by ATP, allowing Ca influx into cells, causing changes in cellular homeostasis. Studies have found that P2RX2 is widely expressed in different tissues and cell types, including germline cells, airway cells, microglia and cardiomyocytes as well as neutrophils, eosinophils, mast cells, T and B lymphocytes. P2RX4 Structure The P2X receptor structure consists of three subunits that form an extended trimer with three ATP binding sites. Subunits of P2X receptors can associate to form homo- or hetero-trimers that differ in their electrophysiological and/or pharmacological properties. Each subunit has an intracellular N- and C-terminus linked by two transmembrane helices (TM1 and TM2) to a large ectodomain that forms an ATP-binding pocket with another P2X ectodomain. Determination of crystal structures of N- and C-terminally truncated zebrafish P2X4 receptors without ATP or with ATP binding revealed the original new fold for each subunit. Binding of extracellular ATP to P2X receptors induces receptor opening, allowing Ca2+ and Na+ entry and K+ efflux. P2RX4 and Disease P2RX4 and Pain Brain-derived neurotrophic factor (BDNF) is expressed in neurons and immune cells. It has multiple roles, including a neurotrophic factor during development and a role in adult learning. BDNF is also implicated in neuropathic pain: a series of studies have shown that de novo P2RX4 expression triggers the release of BDNF from activated spinal microglia, which are the resident macrophages of the central nervous system. Binding of BDNF to neuronal TrkB receptors downregulates the cotransporter KCC2, resulting in a shift in GABA transmission from inhibitory to excitatory, followed by allodynia and mechanical hypersensitivity. Thus, P2RX4 is a mysterious but intriguing candidate for the treatment of pathological pain potential therapeutic targets. Figure 1. Summary of P2RX4 roles in long lasting inflammation and neuropathic pain. (Sarah Lalisse, et al.; 2018) P2RX4 and Sepsis Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. This syndrome is one of the leading causes of death worldwide and is a major public health problem with considerable economic consequences. In 2017, the World Health Organization identified the prevention and treatment of sepsis as a global health priority because the management of the syndrome is complex, including restoration of tissue perfusion with fluids and antimicrobial therapy. The finding of elevated plasma ATP levels in mouse models of sepsis and in patients with sepsis suggests that purinergic receptors may be linked and function in this context. A study of the role of P2X4 in sepsis was conducted using an α-hemolysin-producing E. coli mouse model of infection of sepsis. Results showed that P2X7-deficient or P2X4-deficient mice had lower survival, higher cytokine levels, and activation of intravascular coagulation compared to wild-type controls. Studies have shown that activation of P2X4 and P2X7 is protective against sepsis during infection with uropathogenic E. coli. In addition, another study found that ATP-induced killing of E. coli in infected macrophages was dependent on the production of P2X4 through mitochondrial ROS. These data suggest that P2X4 in macrophages is protective against bacterial dissemination and inflammation in vivo. References 1. Csóka B., et al.; Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight. 2018, 3(11). 2. Zhu G., et al.; Chronic lead exposure enhances the sympathoexcitatory response associated with P2X4 receptor in rat stellate ganglia. Environ Toxicol. 2018, 33(6): 631-639. 3. Aby F., et al.; Inflammatory-induced spinal dorsal horn neurons hyperexcitability is mediated by P2X4 receptors. Pain Rep. 2018, 3(3): e660. 4. Sarah Lalisse, et al.; Sensory neuronal P2RX4 receptors controls BDNF signaling in inflammatory pain. Scientific Reports. 2018, 8(1).
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. This syndrome is one of the leading causes of death worldwide and is a major public health problem with considerable economic consequences. In 2017, the World Health Organization identified the prevention and treatment of sepsis as a global health priority because the management of the syndrome is complex, including restoration of tissue perfusion with fluids and antimicrobial therapy. The finding of elevated plasma ATP levels in mouse models of sepsis and in patients with sepsis suggests that purinergic receptors may be linked and function in this context.
A study of the role of P2X4 in sepsis was conducted using an α-hemolysin-producing E. coli mouse model of infection of sepsis. Results showed that P2X7-deficient or P2X4-deficient mice had lower survival, higher cytokine levels, and activation of intravascular coagulation compared to wild-type controls. Studies have shown that activation of P2X4 and P2X7 is protective against sepsis during infection with uropathogenic E. coli. In addition, another study found that ATP-induced killing of E. coli in infected macrophages was dependent on the production of P2X4 through mitochondrial ROS. These data suggest that P2X4 in macrophages is protective against bacterial dissemination and inflammation in vivo.
References
- Csóka B., et al.; Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight. 2018, 3(11).
- Zhu G., et al.; Chronic lead exposure enhances the sympathoexcitatory response associated with P2X4 receptor in rat stellate ganglia. Environ Toxicol. 2018, 33(6): 631-639.
- Aby F., et al.; Inflammatory-induced spinal dorsal horn neurons hyperexcitability is mediated by P2X4 receptors. Pain Rep. 2018, 3(3): e660.
- Sarah Lalisse, et al.; Sensory neuronal P2RX4 receptors controls BDNF signaling in inflammatory pain. Scientific Reports. 2018, 8(1).
Inquiry
