- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0190 | Human P2RX7 Stable Cell Line-HEK293 | P2RX7 | Human | Epithelial | INQUIRY |
P2RX7 is a unique member of the extracellular ATP (eATP)-gated ion channel family expressed in immune cells, a protein of 595Aa in length. It consists of seven family members: P2X1-7. All family members are trimeric ligand-gated ion channels and display a preference for cations. Their subunits mainly include intracellular N and C termini, two transmembrane domains, and an extracellular domain containing the ATP binding site.
P2RX7, originally described by investigators as an ATP 4-receptor in rat mast cells, formerly also known as the P2Z receptor, is responsible for eATP-dependent lysis of macrophages. P2RX7 has many, many features that make this receptor quite different from other P2Xs. These features include a uniquely low affinity for eATP, and the ability to induce membrane blebbing and cell death. Therefore, P2RX7 is probably best known for its role in regulating innate and adaptive immune responses and is expressed on nearly all cell types of the immune system. The study found that macrophages and microglia express high levels of P2RX7.
Compared to other P2X receptors, P2RX7 possesses an extra-long intracellular C-terminus with an additional 200 amino acids. Mutations at different positions in this region are associated with the loss of various physiological functions. P2RX7 stimulation at low eATP doses opens cation-selective plasma membrane channels permeable to Ca, Na, and K. Chronic/continuous eATP administration reveals a second aspect of P2RX7 signaling, the formation of nonselective macroporous (LP), permeable to molecules up to 900 Da. Therefore, P2RX7 can display the properties of a prototypical cytotoxicity receptor. The inherent insensitivity to eATP and the dire consequences of receptor overstimulation have led to the belief that this low agonist affinity may prevent potentially damaging receptor-mediated inflammation by releasing IL-1β, additional eATP, or other DAMPs Cascade activation safety measures. In fact, although healthy interstitial eATP levels are maintained at nanomole levels or lower, eATP concentrations around sites of inflammation can increase several-fold.
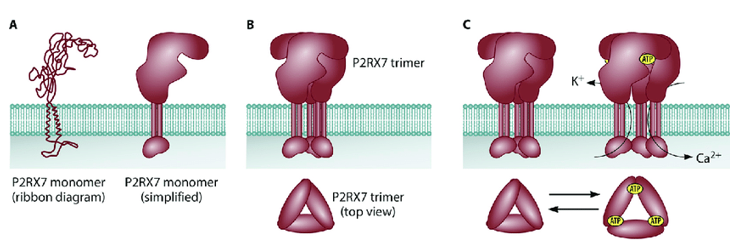
Figure 1. P2RX7 structure and function. (Alexandra Y. Soare, et al.;2021)
Through further investigation, the scientists discovered that P2RX7 function is involved in a variety of physiological processes, including inflammation, proliferation, migration and invasion, metabolism, autophagy, cell death, and neurotransmission. P2RX7 overexpression and overactivation have been implicated in many physiological/pathophysiological processes,including pain, CNS and psychiatric disorders, and cancer.
In addition, the researchers identified a feedback loop in which sustained activation of P2RX7 with LP opening leads to the release of active MMP-2, which stops the reaction through MMP-2-dependent receptor cleavage. This mechanism works in a variety of cells, including macrophages, dystrophic myoblasts, P2RX7-transfected HEK293, and cancer cells. This regulatory mechanism allows malignant cells to benefit from P2RX7 channel activation, triggering proliferation, growth, migration, invasion and metabolic advantages, while avoiding the LP-associated cell death cascade. Furthermore, MMP-2 is present in serum and it exhibits complex regulatory effects through TIMP expression/activity. Therefore, P2RX7 in organs with discontinuous capillaries or in pathologies affecting capillary permeability (eg, inflammation or tumor neovascularization) may be under the regulatory control of MMP-2 cleavage balanced by TIMP. CD44 has been reported to promote eATP binding through GAG chain interactions to allosterically positively regulate P2RX7 activity. CD44 itself was found to be a proteolytic target of MMP-2, suggesting that additional levels of regulatory control may exist. Given that MMP-2 inhibition can reopen P2RX7-LPs in cancer cells and effectively turn on LP-related cell death pathways, it is possible to develop a new generation of cancer therapeutics that promote the formation of this P2RX7-LP.
References
- Adinolfi, E., et al.; Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015, 75, 635-644.
- Alberto, A. V., et al.; Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs. Arch. Pharmacol. 2013, 386, 775-787.
- Al-Khalidi, R., et al.; Zidovudine ameliorates pathology in the mouse model of Duchenne muscular dystrophy via P2RX7 purinoceptor antagonism. Acta Neuropathol. Commun. 2018, 6:27.
- Bartlett, R., et al.; The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014, 66, 638-675.
- Browne, L. E., et al.; P2X7 Receptor channels allow direct permeation of nanometer-sized dyes. J. Neurosci. 2013, 33, 3557-3566.
- Alexandra Y. Soare, et al.; P2RX7 at the Host-Pathogen Interface of Infectious Diseases. Microbiology and molecular biology reviews: MMBR. 2021, 85(1).
Inquiry
