- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- SCN1A
SCN1A
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0047 | Human SCN1A Stable Cell Line-HEK293 | SCN1A | Human | Epithelial | INQUIRY |
| ACC-RI0154 | Human SCN1A Stable Cell Line-CHO | SCN1A | Human | Epithelial-like | INQUIRY |
Vertebrate sodium channels are voltage-gated ion channels, which are essential for the generation and propagation of action potentials, and mainly play a role in nerves and muscles. Analysis of its structure revealed that the voltage-sensitive sodium ion channel is a heteromeric complex composed of a large central pore-forming glycosylated α subunit and two smaller auxiliary β subunits. Functional studies have shown that the transmembrane α subunit of the brain sodium channel is sufficient to express functional sodium channels. Among them, the SCN1A gene provides the coding instructions for the production of the sodium channel α subunit called NaV1.1. These channels transport positively charged sodium ions into the cell and play a key role in the cell's ability to generate and transmit electrical signals. The NaV1.1 channel is involved in transmitting signals from one neuron to another neuron. The neurotransmitter release on which the communication between neurons depends is affected by the flow of sodium ions through the NaV1.1 channel.
SCN1A Features
The scientists determined the coding sequence of the human SCN1A gene by comparing the rat cDNA sequence with the genome sequence. The human SCN1A protein has a sequence of 2,009 amino acids. Further research found that human SCN1A is highly conserved and has 98% amino acid sequence identity with the corresponding rat sequence. Through the analysis of the SCN1A gene structure, it is found that the SCN1A gene has 26 exons.
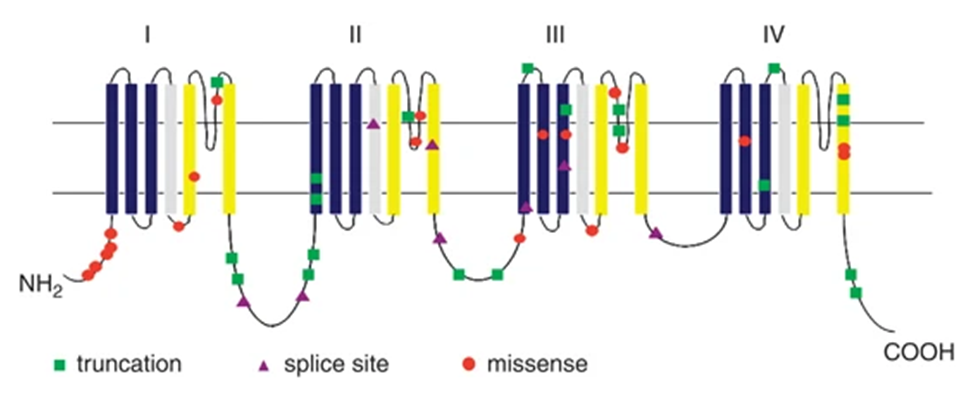
Figure 1. Location of 47 SCN1A point mutations in the sodium channel α1 subunit. (Huihui Sun, et al.; 2010)
SCN1A and Disease
Familial hemiplegic migraine
In patients with familial hemiplegia migraine type 3 (FHM3), at least 7 mutations in the SCN1A gene were found. FHM3 is a type of familial migraine. Each mutation changes a single amino acid sequence in the NaV1.1 channel, thereby changing the structure of the channel. These abnormal channels are open longer than normal, which increases the flow of sodium ions into neurons. This increase causes the cell to release more neurotransmitters. Changes in signals between neurons make FHM3 patients more prone to these severe headaches.
Genetic Epilepsy with Febrile Seizures Plus
Hundreds of mutations in the SCN1A gene that cause hereditary epilepsy with febrile seizures (GEFS+) have been discovered, which is a seizure disorder of varying severity. These symptoms include simple febrile (fever-related) epilepsy, and febrile epilepsy+ (FS+). FS+ includes fever and other types of cramps, including those that are not related to fever (no heat cramps), and these symptoms continue into childhood. The GEFS+ spectrum also includes other conditions, such as Dravet syndrome, which can cause more severe seizures that last longer and may be difficult to control. These recurrent epilepsy will worsen over time and are often accompanied by a decline in brain function.
Mutations in the SCN1A gene, which is the basis of GEFS+, have multiple effects on the function of the NaV1.1 channel. Some mutations change a single amino acid in the channel, thereby changing the structure of the channel. Others result in a non-functional version of NaV1.1 channels, or reduce the number of these channels produced in each cell. There are also mutations that change individual amino acids in key regions of the channel. All these genetic changes affect the NaV1.1 channel's ability to transport sodium ions to neurons. Some mutations are thought to reduce channel activity, while others may increase channel activity. However, it is not yet clear how these genetic changes cause seizures, and why they cause a series of seizure disorders of varying severity.
Other Disorders
A common change (polymorphism) in the SCN1A gene is related to the effectiveness of certain anti-epileptic drugs. This polymorphism is written as ICS5N+5G>a, which changes the nucleotide sequence of a DNA in the SCN1A gene. Studies have shown that this polymorphism is associated with the maximum safe dose of antiepileptic drugs phenytoin and carbamazepine. These drugs treat epilepsy by blocking sodium channels (such as NaV1.1) in neurons. Too small a dose may not be effective in controlling seizures, while too large a dose may cause unnecessary side effects.
References
- Dichgans M, et al.; Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005, 366(9483):371-7.
- Fujiwara T. Clinical spectrum of mutations in SCN1A gene: severe myoclonic epilepsy in infancy and related epilepsies. Epilepsy Res. 2006, 70 Suppl 1:S223-30.
- Gargus JJ, et al.; Novel mutation confirms seizure locus SCN1A is also familial hemiplegic migraine locus FHM3. Pediatr Neurol. 2007, 37(6):407-10.
- Martin MS, et al.; Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010, 285(13):9823-9834.
- Mulley JC, et al.; SCN1A mutations and epilepsy. Hum Mutat. 2005, 25(6):535-42.
- Pietrobon D. Familial hemiplegic migraine. Neurotherapeutics. 2007, 4(2):274-84.
- Escayg, A., et al.; Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nature Genet. 2000, 24: 343-345.
- Huihui Sun, et al.; Analysis of SCN1A mutation and parental origin in patients with Dravet syndrome. Journal of Human Genetics. 2010, 55: 421-427.
Inquiry
