- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0048 | Human SCN2A Stable Cell Line-CHO | SCN2A | Human | Epithelial-like | INQUIRY |
| ACC-RI0157 | Human SCN2A Stable Cell Line-HEK293 | SCN2A | Human | Epithelial | INQUIRY |
The SCN2A gene encodes the voltage-gated sodium channel Na(v)1.2, which plays an important role in the initiation and conduction of action potentials. SCN2A is expressed in the initial segment of axons and Ranvier nodes in myelinated nerve fibers during early development, and later in unmyelinated axons. In many cell types, sodium channels are responsible for the generation and propagation of action potentials, mainly in nerves and muscles. Voltage-sensitive sodium channels are heteromeric complexes composed of a large glycosylated α subunit (approximately 260 kD) and two smaller β subunits (33-39 kD).
SCN2A Cloning and Expression
Studies have found that sodium channels purified from rat brain and skeletal muscle contain one large polypeptide subunit and 2 or 3 smaller polypeptide subunits. In 1986, scientists isolated the cDNA of SCN2A from two different rat brain mRNAs. This cDNA mainly encodes the large peptides of brain sodium channels (designated as I and II). Later, the researchers cloned and sequenced a 1,134 bp fragment from the rat hypothalamic cDNA library, which corresponds to a part of the brain type II sodium channel; this fragment spans the fourth repeat homologous unit in the α subunit, Located between amino acids 1577 and 1960.
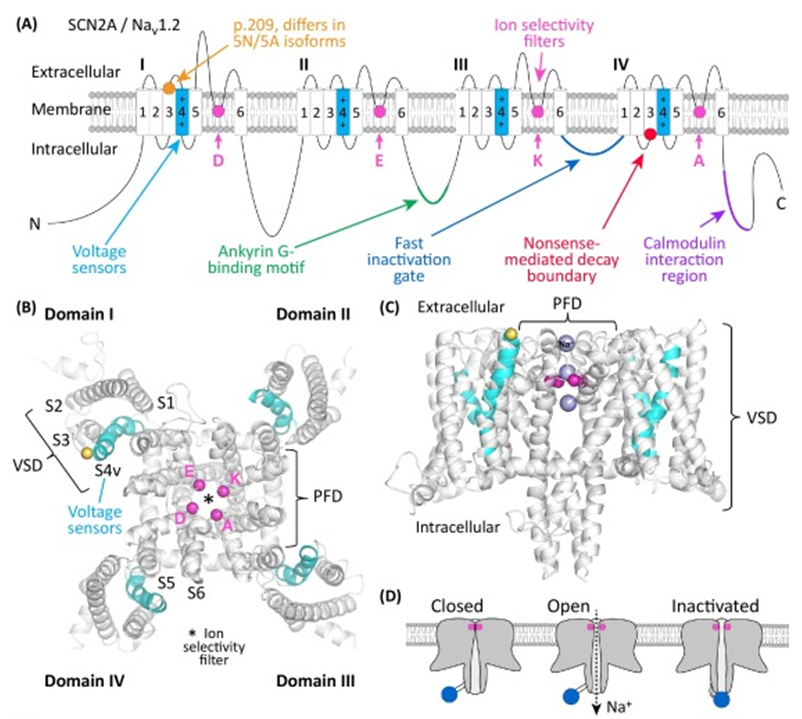
Figure 1. The structure of SCN2A gene.(Stephan J. Sanders, et al.; 2018)
Ahmed et al. used probes derived from human spinal cord sodium channel cDNA to screen the human frontal cortex cDNA library. A full-length cDNA was constructed from 3 overlapping clones corresponding to the sodium channel II α subunit. The predicted protein of 2,005 amino acids has 97% sequence identity with rat brain sodium channel II. Further analysis revealed that the SCN2A protein contains 4 internal homologous repetitive sequences, each repetitive sequence contains 8 potential transmembrane fragments, as well as multiple glycosylation and phosphorylation sites. The transient expression of SCN2A gene in CHO cells showed the voltage dependence of sodium channels, sodium selectivity and tetrodotoxin-sensitive currents, biophysical and pharmacological properties.
SCN2A Gene Function
The researchers evaluated the relative distribution between SCN1A and SCN2A based on the ligase detection strategy. Compared with SCN1A, SCN2A is found to be dominant in the caudal region of the central nervous system. Some non-excitable cells, such as Schwann cells and astrocytes, also express voltage-sensitive sodium channels. In situ hybridization and specific antibody staining proved the expression of type 2 brain sodium channels in embryonic rat osteoblasts, but the expression of sodium channel 1 or fetal sodium channel 3 was not detected.
Further research found that the cytoplasmic loop that connects domains II and III of the NaV1 (SCN2A) subunit contains a determinant that confers compartmentalization in the initial segment of axon of rat hippocampal neurons. The expression of soluble NaV1.2 II-III adaptor protein leads to the disintegration of endogenous sodium channels. This motif is sufficient to redirect somatic dendritic potassium channels to the initial segment of axons, a process that involves association with ankyrin G. Therefore, this motif may play an important role in controlling electrical excitability during development and plasticity.
In addition, the study also found that the SCN2A channel is sensitive to temperature changes; the mutant NaV1.2 channel triggers seizures through functional gain.
SCN2A and Disease
Benign Familial Infantile Seizures
Among the affected members of 6 families with benign familial neonatal and infantile seizures, the researchers identified heterozygous mutations in the SCN2A gene. The three families have the same mutations, which are generated independently based on haplotype analysis.
Developmental and Epileptic Encephalopathy
The researchers identified related heterozygous mutations in the SCN2A gene. Most mutations are missense mutations affecting conserved residues, and there is no obvious correlation between the mutation position and the phenotype. Some patients with late onset and relatively mild phenotypes have nonsense, frameshift, or splice site mutations, which are expected to cause loss of function. Four missense mutations were studied in vitro. Two mutations (V423L and F1597L) observed in patients with severe early-onset epilepsy lead to gain-of-function effects, changes in activation and inactivation curves, and increased current. Two other missense mutations found in children with later seizures are loss-of-function mutations, which result in reduced channel availability and membrane excitability. Ten patients had a mutation in the 1882 amino acid. R1882G causes benign infantile seizures, sometimes accompanied by late-onset ataxia. In contrast, R1882Q, R1882L, and R1882P cause severe mental retardation phenotypes. Other recurrent mutations associated with DEE11 include L1342P and R853Q.
In addition, a de novo heterozygous missense mutation was found in the SCN2A gene. The R1182Q mutation that causes the gain-of-function effect is associated with seizures in the first few days of life, but is not significantly associated with severe abnormal movements. In contrast, the R853Q mutation has been shown to cause a loss-of-function effect of decreased neuronal excitability, which is associated with a high incidence of seizures and involuntary movements between 6 and 8 months, including choreotathotheliosis, muscle Dystonia and cone signs.
Paroxysmal Ataxia Type 9
Liao et al. discovered a de novo heterozygous missense mutation in the SCN2A gene. Electrophysiological studies have shown that the A263V mutation causes a 3-fold increase in sustained sodium current, indicating a significant improvement in function. Other changes include slowing down rapid inactivation and accelerating recovery from slow inactivation. These findings are consistent with gain-of-function effects and neuronal hyperexcitability. In addition, the researchers also identified a de novo mutation of A263V in the SCN2A gene; a heterozygous mutation in the SCN2A gene. In vitro functional electrophysiological studies of transfected cells have shown that the newly reported mutations lead to functional gain effects and increased cell membrane excitability, although studies have shown that there are different molecular mechanisms.
References
- Ahmed, C. M. I., et al.; Primary structure, chromosomal localization, and functional expression of a voltage-gated sodium channel from human brain. Proc. Nat. Acad. Sci. 1992, 89: 8220-8224.
- Berecki, G., et al.; Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy. Proc. Nat. Acad. Sci. 2018, 115: E5516-E5525.
- Berkovic, S. F., et al.; Benign familial neonatal-infantile seizures: characterization of a new sodium channelopathy. Ann. Neurol. 2004, 55: 550-557.
- Black, J. A., et al.; Type II brain sodium channel expression in non-neuronal cells: embryonic rat osteoblasts. Molec. Brain Res. 1995, 34: 89-98.
- Bosmans, F., et al.; Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008, 456: 202-208.
- Cooperman, S. S., et al.; Modulation of sodium-channel mRNA levels in rat skeletal muscle. Proc. Nat. Acad. Sci. 1987, 84: 8721-8725.
- Fazeli, W., et al.; Dominant SCN2A mutation causes familial episodic ataxia and impairment of speech development. Neuropediatrics. 2018, 49: 379-384.
- Garrido, J. J., et al.; A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003, 300: 2091-2094.
- Stephan J. Sanders, et al.; Progress in Understanding and Treating SCN2A-Mediated Disorders. Trends Neurosci. 2018, 41, 7:P442-456.
Inquiry
