- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- SCN3A
SCN3A
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0049 | Human SCN3A Stable Cell Line-CHO | SCN3A | Human | Epithelial-like | INQUIRY |
| ACC-RI0159 | Human SCN3A Stable Cell Line-HEK293 | SCN3A | Human | Epithelial | INQUIRY |
There are more than 350 different ion channels expressed in the mammalian brain, of which 143 are voltage-gated. Gene expression, intracellular calcium ion alternation, and signal processing in neurons are some examples that rely on the activity of voltage-gated ion channels embedded in cell membranes. They play a vital role in cell function, communication and electrical excitability. Gene expression in neurons, intracellular calcium ion alternation, and signal processing are examples of functions that rely on the activity of voltage-gated ion channels embedded in cell membranes. The voltage-gated ion channel superfamily regulates all these physiological processes. This superfamily consists of eight families of voltage-gated sodium, calcium and potassium, calcium-activated potassium channels, inwardly rectified potassium channels, circulating nuclide-regulated ion channels, possible transient receptor channels, and double-hole potassium channels composition. The working mechanism of ion channels can be simply described as when the ion channel is open or active, the pores allow ions to pass through the cell membrane. When they are turned off, ion transmission will stop. Controlling the ion current in neurons promotes these channels to encode, process and transmit neuronal signals (action potentials).
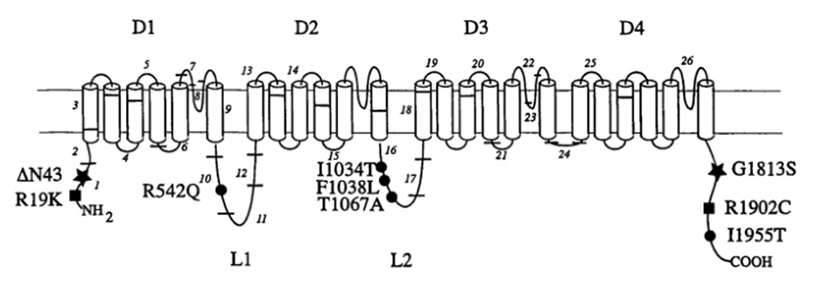
Figure 1. Positions of rare and polymorphic coding substitutions in three sodium channel proteins. (L A Weiss, et al.; 2003)
The structure of the ion channel consists of four variants based on the common structure that forms the pore. Using a high-resolution scanning electron microscope to analyze the structure of the voltage-gated ion channel, the image reconstruction showed that there are four main homology domains (I to IV) surrounding a central hole, indicating the laterally oriented entrance in each region , And the area where ions are transported to the center hole. All these domains are constructed from subunits of transmembrane α-helix.
Voltage-gated Sodium Channel
Voltage-gated sodium (Na+) channels are the main component that promotes the excitability of the cell membrane, because the influx of sodium leads to depolarization of the cell membrane, and therefore the generation and propagation of action potentials in axons. In this regard, they play an important role in the excitability and conductivity of cell membranes in neural networks. In addition, they play a key role in controlling a variety of pain syndromes, including inflammatory pain, neuropathic pain, and central pain associated with spinal cord injury. These channels close quickly when repolarization, or close more slowly when depolarization continues. The voltage-gated Na+ channel consists of an α subunit and an auxiliary β subunit including four homology domains (DI-DIV). Among them, the SCN3A gene encodes the α subunit of the voltage-gated sodium channel Na(v)1.3, which shows rapid activation and inactivation. Voltage-gated sodium channels from the central nervous system are composed of a large pore-forming α subunit, such as SCN3A, which is connected to a smaller auxiliary subunit.
SCN3A Features
Ahmed et al. isolated two cDNAs from the human cerebral cortex library by screening for the existence of specific clones of sodium channel α subunit. One of the clones showed the greatest homology with rat brain sodium channel II. The second clone encodes a different sodium channel subtype, possibly a type III channel. Further research found that the SCN3A gene spans approximately 120 kb and has 30 exons, including a non-coding replacement the first exon, replacement exon 6 and replacement exon 12b.
By using primers based on rat sodium channel cDNA to perform RT-PCR on the frontal pole mRNA, and then screen the human cerebellum cDNA library, the researchers successfully cloned SCN3A. The deduced protein of 1,951 amino acids contains 24 transmembrane domains. Northern blot analysis detected a transcript of approximately 9.5 kb, which was strongly expressed in the brain, weakly expressed in the heart, and not expressed in the placenta, lung, liver, kidney or pancreas. A strong band of about 7.5 kb was also found in skeletal muscle. In specific brain regions, the highest expression of SCN3A was detected in the cerebellum and frontal lobe, moderately expressed in the amygdala, caudate nucleus, hippocampus, substantia nigra, medulla and pole occipital, subthalamic nucleus, thalamus, cerebral cortex, temporal lobe, and Putamen, and not expressed in the corpus callosum and spinal cord.
SCN3A and Disease
Familial Focal Epilepsy
In 4 patients with familial focal epilepsy with variable lesions, the researchers identified 4 different heterozygous missense mutations in the SCN3A gene. These mutations were found through genetic screening of the SCN3A gene in 179 children with SCN1A mutation-negative focal epilepsy. In vitro functional expression studies have shown that mutations cause variable defects in channel function, and only some mutations change the activation and/or inactivation dynamics. However, all abrupt changes resulted in an increase in inward current during the slow depolarization voltage ramp, indicating that channel dysfunction can enhance the response to threshold depolarization input and promote over-excited networks.
Developmental and Epileptic Encephalopathy
In 4 unrelated patients with developmental and epileptic encephalopathy, the researchers identified 3 different de novo heterozygous missense mutations in the SCN3A gene. The mutation was found by exome sequencing and confirmed by Sanger sequencing. Whole-cell voltage-clamp electrophysiological records showed that compared with the control, the slow inactivation/non-inactivation continuous current increase of the mutant channel was consistent with the function gain effect. In addition, there are two variants that cause the voltage dependence of activation on the hyperpolarization potential to shift to the left. Both of these mechanisms are expected to increase the excitability of neurons.
References
- Ahmed, C. M. I., et al.; Primary structure, chromosomal localization, and functional expression of a voltage-gated sodium channel from human brain. Proc. Nat. Acad. Sci. 1992, 89: 8220-8224.
- Chen, Y. H., et al.; Cloning, distribution and functional analysis of the type III sodium channel from human brain. Europ. J. Neurosci. 2000, 12: 4281-4289.
- Kasai, N., et al.; Genomic structures of SCN2A and SCN3A--candidate genes for deafness at the DFNA16 locus. Gene. 2001, 264: 113-122.
- Lamar, T., et al.; SCN3A deficiency associated with increased seizure susceptibility. Neurobiol. Dis. 2017, 102: 38-48.
- Malo, D., et al.; Three brain sodium channel alpha-subunit genes are clustered on the proximal segment of mouse chromosome 2. Genomics. 1991, 10: 666-672.
- Malo, M. S., et al.; Targeted gene walking by low stringency polymerase chain reaction: assignment of a putative human brain sodium channel gene (SCN3A) to chromosome 2q24-31. Proc. Nat. Acad. Sci. 1994, 91: 2975-2979.
- L A Weiss, et al.; Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Molecular Psychiatry. 2003, 8: pages186-194.
Inquiry
