- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0086 | Human SCNN1A/SCNN1B/SCNN1G Stable Cell Line-CHO | SCNN1G | Human | Epithelial-like | INQUIRY |
The SCNN1G gene provides coding instructions for the synthesis of the gamma subunit of a protein complex called the epithelial sodium channel (ENaC). This channel is composed of α, β, and γ subunits, each of which is produced by a different gene. ENaC assembles into a heterotrimer composed of three homologous subunits α, β and γ or δ, β and γ. These channels are located on the surface of certain cells called epithelial cells in many tissues of the human body, including the kidneys, lungs, and sweat glands. In these cells, the ENaC channel transports sodium into the cell. For example, in the kidney, the ENaC channel responds to signals that the blood sodium is too low, allowing sodium to flow into the cell. From the kidney cells, sodium ions are returned to the blood (this process is called reabsorption) instead of being excreted in the urine. Unlike voltage-gated sodium channels, ENaC is constitutively active and independent of voltage. In addition to regulating the amount of sodium in the body, the flow of sodium ions also helps to control the flow of water in the tissues. For example, ENaC channels in lung cells help regulate the amount of fluid in the lungs.
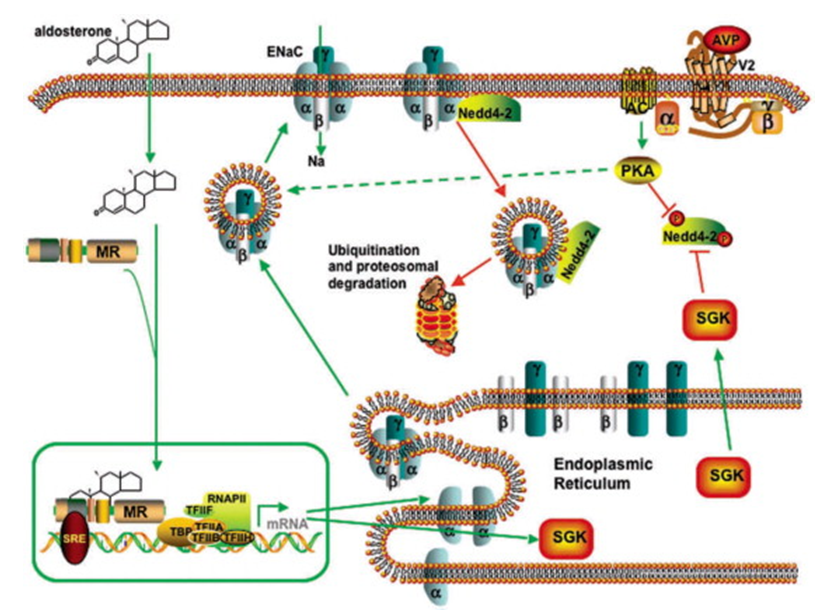
Figure 1.The principal factors that regulate the synthesis, location, and degradation of ENaC are aldosterone and, to a lesser degree, AVP.(Robert J. Alpern, et al.; 2008)
In most vertebrates, sodium ions are the main determinant of the osmotic pressure of the extracellular fluid. ENaC allows sodium ions to transfer across the epithelial cell membrane in so-called "compact epithelial cells" with low permeability. The flow of sodium ions through epithelial cells affects the osmotic pressure of the extracellular fluid. Therefore, ENaC plays a central role in the regulation of body fluid and electrolyte homeostasis, and therefore affects blood pressure. Because ENaC is strongly inhibited by amiloride, it is also called "amiloride-sensitive sodium channel".
SCNN1G Location and Expression
The human gene SCNN1A is located on chromosome 12p, and the human genes encoding SCNN1B and SCNN1G are located in the juxtaposed position on the short arm of chromosome 16 (16p12-p13). The structure of human and rat SCNN1G gene was first reported by Thomas et al. A follow-up study by Saxena et al. reported the complete coding sequence of the human SCNN1G gene and determined that it has 13 exon and intron positions that are conserved in all three human ENaC genes SCNN1A, SCNN1B and SCNN1G. The position of introns is also highly conserved throughout vertebrates.
The three ENaC subunits encoded by SCNN1A, SCNN1B and SCNN1G are usually expressed in compact epithelium with low water permeability. The main organs expressing ENaC include the part of renal tubular epithelium, respiratory tract, female reproductive tract, colon, saliva and sweat glands. In addition, ENaC is also expressed on the tongue and has been proven to be essential for the perception of salt taste. Further studies have found that the expression of ENaC subunit genes is mainly regulated by the mineralocorticoid hormone aldosterone activated by the renin-angiotensin system.
SCNN1G and Disease
Liddle Syndrome
At least five SCNN1G gene mutations can cause Liddle syndrome. People with liddle syndrome have high blood pressure and hypokalemia, usually starting in childhood. Mutations in the SCNN1G gene associated with Liddle syndrome lead to the production of abnormally short proteins in the gamma subunit. These changes affect an important area of the gamma subunit protein, which is involved in the breakdown of signals. As a result of the mutation, the protein is not degraded and more ENaC channels remain on the cell surface. The increase in cell surface channels allows the re-absorption of excess sodium (and subsequently water), leading to high blood pressure. Sodium reabsorption into the blood is related to the removal of potassium from the blood, so excessive sodium reabsorption leads to hypokalemia.
Pseudohypoaldosteronism Type 1
Mutations in the SCNN1G gene are associated with type 1 pseudoaldosteronism (PHA1). This condition usually begins in infancy and is characterized by low sodium and high potassium levels in the blood, as well as severe dehydration due to excessive sodium and fluid loss in the urine. In particular, SCNN1G gene mutation is related to autosomal recessive inheritance of PHA1, which is a serious disease and will not improve with age. Most mutations in the SCNN1G gene result in abnormally short gamma subunit proteins. These mutations result in decreased or missing ENaC channel activity. Therefore, sodium reabsorption is impaired, leading to signs and symptoms of autosomal recessive PHA1 such as hyponatremia. Decreased function of ENaC channels in lung epithelial cells leads to excessive fluid in the lungs and recurrent lung infections.
Other Disorders
Some patients with cystic fibrosis-like syndrome have mutations or normal gene mutations (polymorphisms) in the SCNN1G gene. The signs and symptoms of patients with cystic fibrosis-like syndrome are similar to those of cystic fibrosis, including respiratory problems and lung infections . However, changes in CFTR, the gene most commonly associated with cystic fibrosis, cannot explain the development of the disease. It is believed that genetic mutations in the SCNN1G gene can disrupt sodium transport and fluid balance, leading to the signs and symptoms of cystic fibrosis-like syndrome.
References
- Hanukoglu I, et al.; Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene.2016, 579 (2): 95–132.
- Rossier BC, et al.; Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiological Reviews. 2015, 95 (1): 297–340.
- Ludwig M, et al.; Structural organisation of the gene encoding the alpha-subunit of the human amiloride-sensitive epithelial sodium channel. Human Genetics. 1998, 102 (5): 576–81.
- Enuka Y, et al.; Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochemistry and Cell Biology. 2012, 137 (3): 339–53.
- Masilamani S, et al.; Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999, 104(7):R19-23.
- Mutesa L, et al.; Genetic analysis of Rwandan patients with cystic fibrosis-like symptoms: identification of novel cystic fibrosis transmembrane conductance regulator and epithelial sodium channel gene variants. Chest. 2009, 135(5):1233-1242.
- Snyder PM, et al.; Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995, 83(6):969-78.
- Robert J. Alpern, et al.; Seldin and Giebisch's The Kidney Physiology and Pathophysiology. Academic Press. 2008, ISBN:978-0-12-088488-9.
Inquiry
