- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0193 | Human TRPC1 Stable Cell Line-HEK293 | TRPC1 | Human | Epithelial | INQUIRY |
Classical transient receptor potential channel 1 (TRPC1) belongs to the transient receptor potential (TRP) ion channel superfamily. The latter is a type of non-selective cation channel, which was first discovered in Drosophila, and more than 30 members have been cloned in mammals so far, belonging to seven subfamilies: TRPC, TRPV, TRPM, TRPN, TRPA, TRPPP and TRPML. TRPC1 is the first cloned member of the TRPC subfamily with high homology to the Drosophila TRP channel and is widely distributed in a variety of mammalian tissues. TRPC1 is abundantly expressed in the nervous system and plays a role in various physiological functions and pathological changes, such as neurodevelopment, pain, Parkinson's disease and epilepsy.
Overview of TRPC1 Structure
In 1995, TRPC1 was discovered in mammals for the first time, and it was named Htrp-1. Then in 1996, the functional research results of TRPC1 were also publicly reported, thus officially opened the prelude to the research on TRPC1. The human TRPC1 gene is located on chromosome 3 q22-24, and the genome sequence is 83.84 kb. In humans, four splice variants (α, β, γ, and δ) of TRPC1 mRNA have been found, of which α and β are confirmed to be translated into functional proteins, consisting of 793 and 759 amino acids, respectively. TRPC1 protein is composed of cytoplasmic N-terminal, C-terminal and six transmembrane structural fragments (S1-S6). There is an extracellular loop between S5 and S6. The latter is mainly composed of aspartic acid and glutamic acid and is a negatively charged α-helix structure, which may be the ion-permeable region of TRPC1. There are four ankyrin repeat regions (AAAA) and one helical region (CC) at the N-terminus of TRPC1, which may be involved in the anchoring of channel proteins, and the ankyrin repeat regions may also regulate the homopolymerization of channels. Similar to other TRPC proteins, the C-terminus of TRPC1 has an EWKFAR TRP box structure of unknown function and two calmodulin (CaM) binding sites involved in regulating Ca2+-induced inactivation of TRPC1 currents. In addition, two negatively charged residues at the C-terminus (aspartic acid at 639 and 640) can interact with positively charged residues (lysine at 684 and 685) on calcium sensor-substrate interacting molecule 1 (STIM1) acid) to regulate the opening and closing of TRPC1 channels. In recent years, studies have found that the translation of human TRPC1 starts from the upstream leucine more efficiently, that is, an increase of 78 amino acids upstream of the known TRPC1, which may be a protein form closer to normal TRPC1 than short TRPC1. Examination of this N-terminal extension revealed the presence of a glycine-serine residue, suggesting that there may be a highly elastic hinge region connecting the N-terminal extension and the rest of the structure; and there is also a positive charge upstream of this elastic hinge region residues, these results suggest that the N-terminal extension is likely to be an important sequence in TRPC1 ion channel function. A single TRPC1 protein cannot form an ion channel. A functional TRPC1 channel is usually a heterotetramer composed of TRPC1 and other TRP subunits, such as TRPC3, 4, 5, 6, and 7.
Regulation of TRPC1
Receptor Regulation Activates TRPC1 Channel
TRPC1 can combine with other TRP subunits to form receptor-regulated and activated channel complexes. For example, in transfected cells and adult rat brain tissue, the combination of TRPC1 and TRPC5 was found to change the activity of TRPC5 in a voltage-dependent manner. When the receptor-regulated activation current can be generated. Similarly, expression of endogenous TRPC1, 2, 5, and 6 was found in gonadotropin-releasing hormone (GnRH) neurons, and these channels were activated by carbachol via Gq-coupled protein to trigger Ca2+ influx, but this effect was significantly reduced after down-regulating the expression of TRPC1. The above results indicate that functional homologous TRPC1 tetramers may not exist, and TRPC1 can only form functional heterotetrameric ion channels activated by receptor regulation together with other TRP subunits. However, studies using lipid bilayer remodeling and atomic force spectroscopy found that TRPC1 can also form homotetramers on the cell membrane, but its function has not yet been determined.
Calcium Store Emptying Activates TRPC1 Channel
Recent studies have confirmed that when STIM1 on the endoplasmic reticulum senses that the calcium store is emptied, it respectively binds to calcium release-activated calcium modulator 1 (CRACM1 or Orai1) and TRPC1 on the cell membrane, and the activated Orai1 can promote the expression of TRPC1 on the cell membrane. So STIM1 can regulates the opening of TRPC1 channels, allowing a large amount of Ca2+ influx. Therefore, TRPC1, STIM1 and Orai1 together constitute a calcium store-operated calcium channel. In the study of mouse retinal Muller cells, it was found that the pool-operated Ca2+ response triggered by the synergistic activation of TRPC1 and Orai1 plays an important role in key retinal structures, signaling pathways, osmoregulation and mechanosensory functions. These results suggest a role for TRPC1 in library-operated Ca influx.
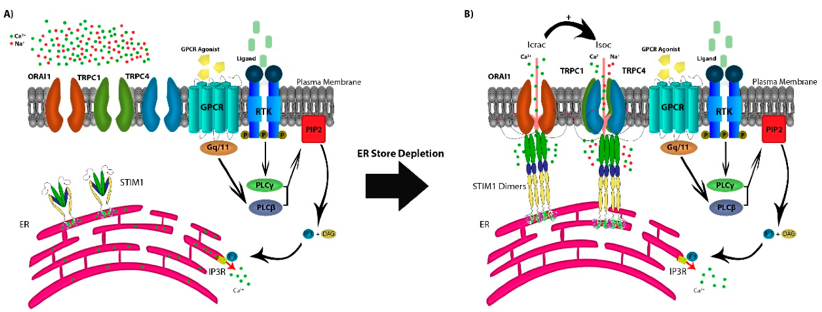
Figure 1. The store-operated Ca2+ entry pathway. (Osama M Elzamzamy, et al.; 2020)
Mechanical Force Activates TRPC1 Channels
The TRPC1 protein expressed on the axonal growth cone of neurons terminates the growth of axons by activating the downstream calpain, which may be related to the mechanosensation of touch objects and the adjustment of axonal growth direction during axonal growth. In the cystitis model induced by the cytostatic cyclophosphamide, the expression of TRPC1 was increased in DRG sensory neurons innervating the bladder, and a large number of budding terminals appeared in the bladder mucosa. This phenomenon was observed in TRPC1 and TRPC4 knockout mice. In addition, the bladder of the knockout mice no longer showed obvious irritability, suggesting that TRPC1 may be an important molecule in bladder mechanosensation.
Physiological Function of TRPC1
The expression of TRPC1 in the embryonic central nervous system is higher than that in adults. Studies have confirmed that after silencing TRPC1 expression, the differentiation and neurite outgrowth of mouse embryonic neural stem cells under the action of electromagnetic fields will be significantly reduced, suggesting that TRPC1 may be involved in early neuron development and proliferation. TRPC1 expressed in the hippocampal and spinal neurons of Xenopus laevis can regulate the chemotaxis or repulsion of neuronal growth cones by nerve growth factor or myelin-related glycoprotein, indicating that TRPC1 is involved in the guidance of neuronal processes in early development. In addition, the coexistence of TRPC1 and mGluR1 also exists in neurons of the auditory brainstem, suggesting the role of TRPC1 in the mGluR pathway and midbrain auditory signal transmission.
References
- Zeng C, et al.; Trpc channels: prominent candidates of underlying mechanism in neuropsychiatric diseases. Mol Neurobiol, 2016, 53: 631- 647.
- Ong EC et al.; A Trpc1 protein-dependent pathway regulates osteoclast formation and function. J Biol Chem. 2013, 288: 22219-22232.
- Storch U, et al.; Transient receptor potential channel 1 (Trpc1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012, 287: 3530-3540.
- Osama M Elzamzamy, et al.; The Role of TRPC1 in Modulating Cancer Progression. Cells. 2020, 9(2):388.
Inquiry
