- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Sodium Channels
- Potassium Channels
- Chloride Channels
- Calcium Channels
- TRP Channels
- ATP gated P2X Channels
- ASICs
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Glycine Receptors
- 5-HT Receptors3
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
- TRPC6
TRPC6
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0194 | Human TRPC6 Stable Cell Line-HEK293 | TRPC6 | Human | Epithelial | INQUIRY |
Transient receptor potential (TRP) channels are a class of voltage-independent cation channels that were first discovered in the study of visual transduction in Drosophila. In mammals, TRP channels are mainly divided into seven subfamilies: TRPC, TRPV, TRPM, TRPML, TRPN, TRPA, and TRPP. TRPC subfamily includes 7 members: TRPC1, TRPC2, TRPC3, TRPC4, TRPC5, TRPC6, TRPC7; TRPV subfamily includes 6 members: TRPV1, TRPV2, TRPV3, TRPV4, TRPV5, TRPV6; TRPM subfamily includes 8 members: TRPM1, TRPM2, TRPM3, TRPM4, TRPM5, TRPM6, TRPM7, TRPM8; the TRPML subfamily includes 3 members: TRPML1, TRPML2, TRPML3; and the specific typing of the TRPN subfamily is not yet clear, and only TRPA1 is currently found in the TRPA subfamily; TRPP subfamily includes five members: TRPP1, TRPP2, TRPP3, TRPP4, TRPP5. Among them, TRPC6 is widely distributed in the body, participates in the pathophysiological process of various diseases, and plays an important role in the occurrence and development of diseases.
Structure and physiological function of TRPC6
Structure of TRPC6
TRPC6 consists of 931 amino acids, is expressed on the cell membrane and has 6 transmembrane domains (TM1-6), and there is a functional pore domain between the fifth and sixth transmembrane regions, which constitutes a non-selective region cation channels. The N-terminus and C-terminus of TRPC6 structure are both located in the cell, and the C-terminus tail contains a calmodulin IP3 receptor phosphoinositide-binding site (CIRPIB), CIRPIB is the site of protein interaction and may be involved in the activation of TRPC6. TRPC6 is a non-selective cation channel, it can be directly activated by phospholipase C (PLC), diacylglycerol (DAG), 1-oleoyl-2-acetyl-sn-glycerol, tyrosine protein kinase, 20-hydroxyeicosan, tetraenoic acid, tyrosine kinase, and calmodulin kinase II. In addition, mechanical stimulation, changes in osmotic pressure and pH can also indirectly activate TRPC6, thereby making it involved in the pathophysiological process of various diseases.
Physiological Functions of TRPC6
The main physiological function of TRPC6 is to regulate Na+, K+, Ca2+ and other cation signal transduction, and the Ca2+ imbalance caused by its abnormal function is an important factor for the abnormal physiological function of the cardiovascular system. Studies have found that TRPC6 is complexly regulated by intracellular and extracellular Ca2+, calmodulin and phosphatidylinosine, as well as protein serine and tyrosine phosphorylation. TRPC6 is expressed in vascular smooth muscle, activated TRPC6 can regulate vascular tone through G protein-coupled receptors and tyrosine kinase receptors. Studies have shown that TRPC6 is also involved in the process of hypoxic pulmonary vasoconstriction, the recovery of phagosome activity, the occurrence of autophagy, platelet activation and tumor Changes in cell homeostasis. In addition to the above physiological functions, TRPC6 is also involved in the pathophysiological process of various diseases.
TRPC6 and Disease
TRPC6 is widely present in the cardiovascular system, mainly expressed in cardiomyocytes, fibroblasts, vascular endothelial cells, and vascular smooth muscle cells (VSMC), etc. play an important role in. In addition, studies have also found that TRPC6 is abnormally expressed in various malignant tumors such as prostate cancer, liver cancer, breast cancer, gastric cancer, non-small cell lung cancer, B lymphoma, and is related to the occurrence and development of these cancers.
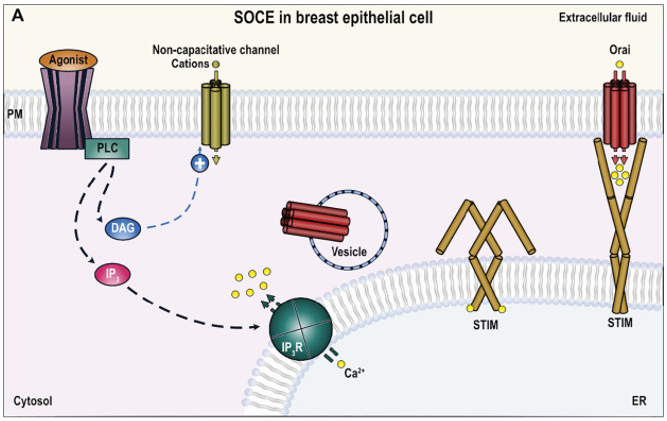
Figure 1. Role of TRPC6 in breast cancer cells. (Isaac Jardin, et al.; 2020)
TRPC6 and MIRI
Myocardial ischemia-reperfusion injury (MIRI) is a common complication of ischemic myocardium after restoration of blood flow, and TRPC6 may be involved in the occurrence of MIRI. In a MIRI study in Wistar male rats, it was found that short-term high-intensity training (HIIT) increased plasma levels of Klotho (an aging-related protein that promotes repair of vascular damage) and decreased TRPC6 channels protein expression during ischemia-reperfusion. These can reduce myocardial infarction size, thereby enhancing the protective effect on ischemia-reperfusion heart.
TRPC6 and Cardiac Hypertrophy
Cardiac hypertrophy is a compensatory response of cardiomyocytes, manifested by increased cardiomyocyte volume, increased cardiomyocyte apoptosis, and aggravated interstitial fibrosis. When cardiomyocytes are decompensated, they develop into heart failure (HF). Abnormal CaN-NFAT pathway regulated by Ca2+ is a key factor leading to cardiac hypertrophy, and the activation of Ca2+ storage-operated calcium channel (SOCE) in cardiomyocytes is related to the expression level of TRPC6.
TRPC6 and Myocardial Fibrosis
Fibroblasts are the main players in the process of myocardial fibrosis, and their transformation into myofibroblasts plays an important role in the production of extracellular matrix proteins, which are closely related to cardiac fibrosis. Studies have shown that Galpha12/13-mediated upregulation of TRPC6 channels is involved in sustained Ca2+ influx and NFAT activation, and negatively regulates endothelin-1 (ET-1)-induced collagen synthesis in myofibroblasts, thereby inhibit myocardial fibrosis.
TRPC6 and Cardiac Hypertrophy
Cardiac hypertrophy is a compensatory response of cardiomyocytes, manifested by increased cardiomyocyte volume, increased cardiomyocyte apoptosis, and aggravated interstitial fibrosis. When cardiomyocytes are decompensated, they develop into heart failure (HF). Abnormal CaN-NFAT pathway regulated by Ca2+ is a key factor leading to cardiac hypertrophy, and the activation of Ca2+ storage-operated calcium channel (SOCE) in cardiomyocytes is related to the expression level of TRPC6.
TRPC6 and Myocardial Fibrosis
Fibroblasts are the main players in the process of myocardial fibrosis, and their transformation into myofibroblasts plays an important role in the production of extracellular matrix proteins, which are closely related to cardiac fibrosis. Studies have shown that Galpha12/13-mediated upregulation of TRPC6 channels is involved in sustained Ca2+ influx and NFAT activation, and negatively regulates endothelin-1 (ET-1)-induced collagen synthesis in myofibroblasts. Inhibit myocardial fibrosis.
TRPC6 and Hypertension
The role of TRPC6 in the mechanism of essential hypertension is related to the regulation of intracellular Ca2+ concentration in VSMCs, and abnormal Ca2+ activity is one of the important factors in the occurrence of hypertension. Upregulation of TRPC6 expression can lead to the transformation of VSMCs to a pathological phenotype, and the use of TRPC6 inhibitors reverses this change and improves hypertension in hypertensive model animals.
TRPC6 and Arrhythmias
TRPC6 is involved in the occurrence of non-valvular atrial fibrillation, and the mechanism may be that TRPC6 and SOCE promote cardiac cardiac electrical remodeling by promoting spontaneous Ca2+ influx in cardiomyocytes, which in turn triggers arrhythmias. Atrial mechanical stretch response can activate endocardial TRPC6, causing atrial arrhythmias, and the mechanism may be to activate TRPC6, increase calcium load in ventricular myocytes, and then trigger arrhythmias.
TRPC6 and Atherosclerosis
Studies have shown that the coronary arteries of pigs with metabolic syndrome exhibit marked atherosclerosis. TRPC6 in coronary smooth muscle cells of pigs is up-regulated, and long-term use of the mineralocorticoid receptor inhibitor spironolactone significantly down-regulates TRPC6 expression in coronary smooth muscle cells, and inhibits excessive coronary constriction and atherosclerosis. In addition, activated monocytes adhere to the arterial endothelium and migrate to the endothelium, which is crucial for the early pathogenesis of atherosclerosis, and up-regulation of TRPC6 can promote the activation of monocytes, thereby promoting atherosclerosis development.
References
- EI Boustany C, et al.; Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008, 47(6): 2068-2077.
- Thebault S, et al.; Differential role of transient receptor potential channels in Ca2 + entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006, 66(4): 2038-2047.
- Tsagareli M, Nozadze I. An overview on transient receptor potential channels superfamily. Behav Pharmacol. 2020, 31(5): 413-34.
- Tang Q L, et al.; Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res. 2018, 28(7): 746-55.
- Inoue R, et al.; The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001, 88(3): 325-32.
- Sanchez C J, et al.; Functional role of TRPC6 and STIM2 in cytosolic and endoplasmic reticulum Ca2+ content in resting estrogen receptor-positive breast cancer cells. Biochem J. 2020, 477(17): 3183-97.
- Isaac Jardin, et al.; TRPC6 channel and its implications in breast cancer: an overview. 2020, Volume 1867, Issue 12, 118828.
Inquiry
