- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0196 | Human TRPM4 Stable Cell Line-CHO | TRPM4 | Human | Epithelial-like | INQUIRY |
| ACC-RI0198 | Human TRPM4 Stable Cell Line-HEK293 | TRPM4 | Human | Epithelial | INQUIRY |
Transient receptor potential (TRP) channels are a superfamily, activation of TRP channels usually produces depolarizing currents, and thus these channels are critical in excitable cells. Transient receptor potential M (Transient receptor potential melastatin, TRPM) has the largest structure in this family, and its member TRPM4 is a non-selective cation channel activated by Ca2+, which can be activated by elevated Ca2+ in the cytoplasm but not through Ca2+. This channel is mainly selective for Na+ and K+. TRPM4 is distributed in many tissues and organs and is involved in complex physiological and pathological mechanisms, mainly related to calcium-dependent mechanisms, such as insulin secretion, immune response, respiratory response, tumor development and cardiovascular disease. In the heart, the TRPM4 channel is the only arrhythmia-related pathway in the TRP family. It is abundantly expressed in cardiomyocytes and special cells of the cardiac conduction system, and is associated with processes such as action potential and cardiac remodeling, and there are species differences.
Structure of the TRPM4 Channel
TRPM4 channel is a member of the TRP channel family and the only calcium-activated non-selective (CAN) cation channel in cardiomyocytes so far. As a transmembrane protein containing 1214 amino acids, TRPM4 is located at 19q13.33 in humans with a genome span of 54kb. The tertiary structure of the ion channel is mainly composed of the TRPM homology region (MHR1-4), Pre-S1 elbow, Pre-S1 shoulder, transmembrane region S1-S6, TRP domain and C-terminal helix (CH1-3) and so on. A pore-forming region exists between the transmembrane domains S5 and S6 of TRPM4, which is selective for passing ions and allows the passage of monovalent cations. It was previously reported that TRPM4 tetramer formation is influenced by the formation of the N-terminal nucleotide-binding domain and the coiled-coil at the C-terminal end. The amino and carboxy terminal regions of TRPM4 contain binding sites associated with channel activation, the ATP-binding domain and N-terminal nucleotide binding domains are associated with inhibition of TRPM4 channels. In addition, there are several phosphorylation sites of protein kinase A (PKA) and protein kinase C (PKC), 5 calmodulin binding sites, 4 Walker B motifs (presumably is an ATP-binding site), a phosphatidyl inositol 4,5-bisphosphate (PIP2) binding site, and a coiled-coil domain, all of which are involved in the regulation of TRPM4 function . The scientists used single-particle electron cryomicroscopy to analyze the structure of the TRPM4 ion channel in the calcium-bound and unbound states. By comparing the difference between the two structures, it was pointed out that there is a clear calcium-binding site in the S1-S4 region of the cell, and the binding of Ca2+ causes a conformational change, which in turn leads to the opening of voltage-dependent channels. Like many other TRP family ion channels, TPPM4 channels also exhibit voltage sensitivity, and studies have shown that Ca2+-activated TRPM4 currents exhibit significant outward rectifying currents with a stepwise increase in voltage. After TRPM4 channels are activated, Na+ enters the membrane to depolarize the plasma membrane, which in turn increases the influx of Ca2+ through other calcium channels, or otherwise regulates the concentration of Ca2+.
Regulation of TRPM4 Channels
Activation of TRPM4 as a channel related to Ca2+ and voltage activation, TRPM4 channels are activated in various ways, and the main view is currently Ca2+ sensitivity regulation and compound activation. Vennekens identified five calmodulin-binding sites in TRPM4 and showed that deletion of any of the three C-terminal sites reduces calcium sensitivity, shifting the voltage-dependent activation of activation to very positive potentials that severely Attenuate current activation. The regulation is mainly affected by PIP2, ATP, PKC-dependent phosphorylation and the binding of calmodulin (CaM) to the C-terminus of TRPM4. Among them, PIP2 is a potent enhancer of TRPM4, which may desensitize the activity of TRPM4 through the decomposition of PIP2 mediated by phospholipase C and then act. It cannot activate TRPM4 by itself, but can correct the desensitization of TRPM4, increase the sensitivity of TRPM4 to Ca2+, and inhibit the voltage dependence of TRPM4. ATP has a dual effect on TRPM4, ATP ionization will block the channel, and its binding may have the effect of stabilizing or restoring the channel's Ca2+ sensitivity; PKC phosphorylation will enhance the sensitivity of TRPM4 to Ca2+; The CaM-binding mutant does not affect TRPM4 trafficking and transport to the plasma membrane, but the C-terminal CaM-binding site is essential for TRPM4's Ca sensing. Two compounds that can activate TRPM4 ion channels have also been found, namely Decavanadate (DV) and 3,5-bistrifluoromethyl pyrazole derivative (BTP2). DV is a compound that can interfere with ATP-dependent transporters. DV does not antagonize the ATP blocker of TRPM4, but acts as a channel activator by inhibiting voltage-dependent channel closing, and has a negative effect on TRPM4. BTP2 is a pyrazole compound that reduces the driving force for Ca2+ entry and inhibits TRPM4 channels at low nanomolar concentrations.
TRPM4 and Cardiovascular Disease
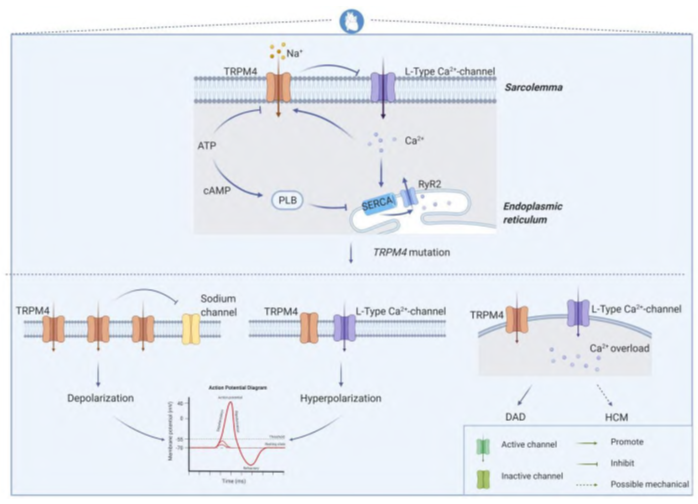
Figure 1. Overview of TRPM4 mutations leading to cardiovascular disease.
In the heart, using a Trpm4 knockout mouse model, this ion channel has been shown to be involved in multiple processes including β-adrenergic regulation, cardiac conduction, action potential duration, and hypertrophic adaptation. In a physiological setting, the TRPM4 channel is directly activated when intracellular Ca2+ rises and ATP levels fall, and in cardiomyocytes it also functions as a negative regulator of L-type calcium currents. This arrangement is critical for the tight functional cooperation between calcium channels and ryanodine receptors, thereby driving excitation-contraction coupling in the heart. Changes in the number or open/closed state of the ion channel affect the membrane transport of Na+ or Ca2+, which in turn affects the depolarization or hyperpolarization process of cardiomyocyte action potentials. When calcium overload exists in cardiomyocytes, it can also lead to delayed post-depolarization or hypertrophic cardiomyopathy. In the heart, TRPM4 channel signaling levels are higher in atrial cardiomyocytes than in normal ventricular cells, highest in Purkinje fibers and less distributed in subendocardial and rare intramural cell bundles.
References
- Hof T, et al.; Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol. 2019, 16(6): 344-360.
- Autzen HE, et al.; Structure of the human TRPM4 ion channel in a lipid nanodisc. Science. 2018, 359(6372):228-232.
- Launay P, et al.; TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002,109(3): 397-407.
- Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007, (179):269-285.
- Nilius B, et al.; Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004, 560(Pt 3):753-765.
Inquiry
