- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0199 | Human TRPM8 Stable Cell Line-CHO | TRPM8 | Human | Epithelial-like | INQUIRY |
Transient receptor potential (TRP) is a voltage-independent cation channel that is widely expressed in a variety of mammalian tissues and participates in many important physiological functions of the body, including muscle contraction, transmitter release, cell proliferation and differentiation, apoptosis and cell death. TRPM8 is a member of the TRP superfamily and is a thermoreceptor that can be activated by different factors, including voltage, pH changes, various chemical substances, cold stimulation, lipid complexes and other endogenous ligands, etc. It penetrates Ca2+, Mg2+ and other cations, and regulate a series of physiological and pathological processes of the organism, such as cell proliferation, migration and apoptosis, the body's inflammatory response, cold sensation, pain sensation, tumor, blood vessels and muscle tension, etc.
TRPM8 Structure and Characteristics
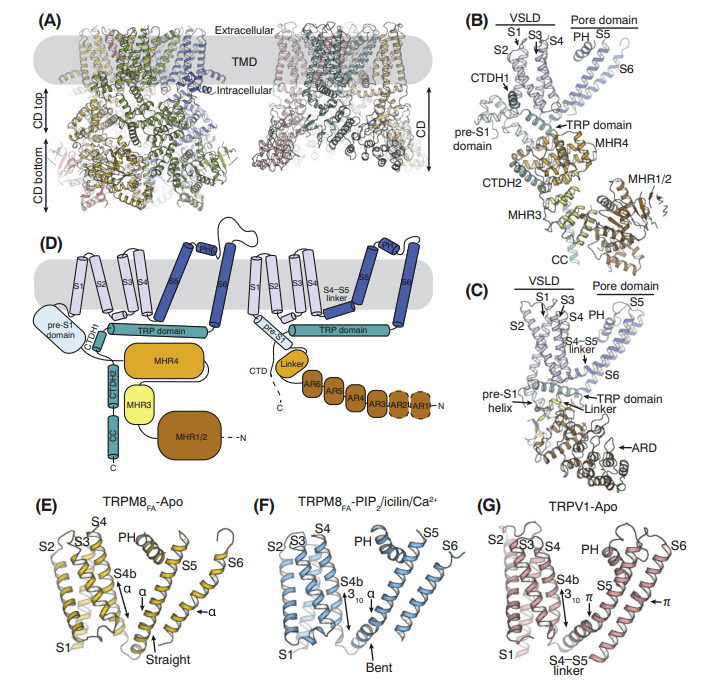
Figure 1. Unique Structural Features of the TRPM8.
TRPM8 was first discovered in the prostate and can be activated by cold environment (8 ℃-28 ℃), menthol, borneol and other cool substances, also known as cold and menthol receptor (CMR1), TRPM8 mRNA or protein mainly exists in the back. In the sensory neurons of root ganglion and trigeminal nerve, recent studies have reported that TRPM8 is also expressed in oral cavity, skin, sperm, tumor, etc. The TRPM8 gene is located on chromosome 2 with a full length of 102.12 kb. The mature mRNA encodes a protein with 1104 amino acid residues and a molecular mass of about 130 Kd. The exact structure of TRPM8 has not yet been resolved. The basic structure contains 6 transmembrane helical fragments (S), and there is a hydrophobic pore loop structure between S5 and S6. Both the N-terminus and C-terminus are in the cell membrane or intracellular organelle membrane. Fluorescent Imaging Plate Reader (FLIPR) and mutation experiments have confirmed that the S2 of TRPM8 is the key domain of menthol; the study also found that the cooling activity of menthol is mainly affected by van der Waals forces, electrostatic and hydrophobic/hydrophilic forces during the action of TRPM8. Its action site in TRPM8 is the active cavity formed by amino acids such as Asn741, Val742, Val743, Phe744, Tyr745, Ile746, Ala747, 107Phe748, Leu749 and Leu750 in the S2 helical chain.
TRPM8 is a direct-gated channel of cold and chemical agonists, and its gating-dependent PIP2 is the result of its direct interaction with lipids. The activation of TRPM8 by menthol is different from that of cold stimulation and borneol. The effect of borneol depends on the presence of Ca2+ in cellular proteins. Menthol increases the potency of PIP2-activated channels and also increases the connection of inositol phosphate channel proteins. Another study found that the activation of TRPM8 by borneol and cold stimulation was regulated by intracellular pH, but menthol was not affected by pH. The activation of TRPM8 by human spermatozoa to increase Ca2+ under the action of temperature or menthol can be inhibited by BCTC and capsazepine, while the acrosome movement induced by progesterone and ZP3 cannot be blocked by BCTC and capsazepine. All of these can indicate that TRPM8 is activated by different signaling pathways.
Pathophysiology of TRPM8
Research on Cold Sense
TRPM8 is mainly distributed in neurons such as trigeminal nerve (TG) and peripheral dorsal root nerve (DRG), and these two parts are the main locations of cold sensation. TRPM8 can be affected by menthol, icilin and cold environment (8-28 ℃). activation, all of which indicate that TRPM8 is a cold receptor. TRPM8 transports the plasma membrane LAMP1 and VAMP7 mediate the fusion of these vesicles with the plasma membrane. VAMP7-deficient mice show reduced functional expression of TRPM8 in sensory neurons and reduced cold-avoidance-induced cold hypersensitivity. Activation of TRPM8 channels on the plasma membrane causes transient accumulation of TRPM8-containing vesicles on the cell surface, which in turn causes a transient increase in the number of functional channels, which further affects the intrinsic properties of cold receptor responses, indicating that the cold sensation of TRPM8 is closely related to intact membranes bubble transport. Studies have shown that TRPM8 is expressed in primary cultured cells, human dental pulp cells (HPCs), mouse odontoblasts, and dentin, suggesting that TRPM8 may be involved in the cold sensor of teeth.
Research on Pain Sense
Some studies have pointed out that TRPM8 may have two subgroups: one is sensitive to menthol and insensitive to capsaicin, ATP and acid, called MS/CIS, which experiments show that it is a cold sensory receptor;the other is sensitive to menthol, capsaicin, ATP and acid stimuli, called MS/CS, and has been shown to be a pain receptor. TRPM8 is much higher in MS/CIS neurons than in MS/CS neurons, indicating that TRPM8 is not only involved in cold sensation, but also in Pain perception and regulation. Some studies have also pointed out that TRPM8 can not only sense cold perception, but also participate in the pain regulation of inflammation and neuropathic pain after being stimulated by moderately low temperature or cold substances, which can be used as a new target for neuropathic pain treatment. Some studies have used resinous toxin (RTX) pretreatment to perform spinal dorsal nerve ligation (SNL) in rats, which can significantly reduce heat and cold hypersensitivity reactions, and found that SNL reduced the expression level of TRPM8, and concluded that the analgesic effect of nervous system nerves. , especially hypersensitivity to cold, may be related to the inhibition of TRPM8 expression in the dorsal root ganglia.
Research in Oncology
TRPM8 is closely related to malignant tumors. In recent years, studies have found that TRPM8 is expressed or even highly expressed in prostate cancer, breast cancer, bladder cancer and other tumors. More importantly, it is widely expressed in tumor tissues, which is likely to be a carcinogenic gene or tumor-initiating gene, and some scholars even call TRPM8 an oncogene; other studies have pointed out that ion channels can be used as potential anti-tumor targets. TRPM8 was first discovered in the prostate, and TRPM8 is closely related to prostate cancer. Some studies have shown that changes in calcium homeostasis are involved in carcinogenesis, and the calcium-permeable channels of TRPM8 are differentially regulated in the process of prostate carcinogenesis, and are involved in prostate cancer cell proliferation, survival, and cell migration. Prostate-specific antigen (PSA) is considered to be a physiological receptor agonist of TRPM8. When PSA activates TRPM8, the activity of PC3 in prostate cancer cells decreases. TRPM8 has a protective effect on the aggressiveness of prostate cancer cells. Studies have shown that TRPM8 overexpression significantly inhibits PC3 cell migration, and this inhibition occurs through inactivation of focal adhesion kinase. Some studies have pointed out that the expression of TRPM8 in prostate cancer cells is regulated by androgen, but not in androgen-independent cell lines. Another study also pointed out that the effect of TRPM8 on prostate cancer cell viability is regulated by androgen and differential expression of TRPM8 isoforms in prostate cancer cells. TRPM8 inhibitor BCTC acts on prostate cancer DU145 cells, blocking TRPM8 can inhibit the proliferation, migration and invasion of prostate cancer cells DU145, but has no effect on normal prostate epithelial cells, which provides a new idea for cancer treatment.
References
- Bai V U, et al; Androgen regulated TRPM8 expression: a potential mRNA marker for metastatic prostate cancer detection in body fluids. International Journal of Oncology. 2010, 36( 2) : 443-50.
- Abe J, et al.; TRPM8 protein localization in trigeminal ganglion and taste papillae. Molecular Brain Research. 2005,136( 1-2) : 91-98.
- De Blas G A, et al.; TRPM8,a Versatile Channel in Human Sperm. Plos One. 2009, 4 ( 6 ) : e6095-e6095.
- Yin Ying, et al.; Current View of Ligand and Lipid Recognition by the Menthol Receptor TRPM8. Trends in Biochemical Sciences. 2020, VOLUME 45, ISSUE 9, P806-819.
Inquiry
