- Home
-
Screening
- Ionic Screening Service
-
Ionic Screening Panel
- Ligand Gated Ion Channels
- Glycine Receptors
- 5-HT Receptors3
- Nicotinic Acetylcholine Receptors
- Ionotropic Glutamate-gated Receptors
- GABAa Receptors
- Cystic Fibrosis Transmembrane Conductance Regulators (CFTR)
- ATP gated P2X Channels
- Voltage-Gated Ion Channels
- Calcium Channels
- Chloride Channels
- Potassium Channels
- Sodium Channels
- ASICs
- TRP Channels
- Other Ion Channels
- Stable Cell Lines
- Cardiology
- Neurology
- Ophthalmology
-
Platform
-
Experiment Systems
- Xenopus Oocyte Screening Model
- Acute Isolated Cardiomyocytes
- Acute Dissociated Neurons
- Primary Cultured Neurons
- Cultured Neuronal Cell Lines
- iPSC-derived Cardiomyocytes/Neurons
- Acute/Cultured Organotypic Brain Slices
- Oxygen Glucose Deprivation Model
- 3D Cell Culture
- iPSC-derived Neurons
- Isolation and culture of neural stem/progenitor cells
- Animal Models
- Techinques
- Resource
- Equipment
-
Experiment Systems
- Order
- Careers
- Home
- Symbol Search
| Catalog | Product Name | Gene Name | Species | Morphology | Price |
|---|---|---|---|---|---|
| ACC-RI0057 | Human TRPV1 Stable Cell Line-HEK293 | TRPV1 | Human | Epithelial | INQUIRY |
The transient receptor potential vanilloid receptor (TRPV1) is one of the important members of the transient receptor potential (TRP) superfamily. It is an important non-selective cation channel mainly located on the cell membrane. Studies have shown that TRPV1 is a molecular integrator of sensory nerves that mediate certain noxious stimuli, and is closely related to the production of inflammatory pain. A variety of neuroinflammatory mediators and endogenous mediators can directly or indirectly activate TRPV1. After TRPV1 is activated, it will cause extracellular calcium ions to influx, which will excite sensory neurons, release excitatory amino acids and a variety of neuropeptides, and trigger related biological effects.
Distribution of TRPV1
TRPV1 is mainly distributed in the body of small and medium primary sensory neurons in peripheral ganglia, as well as non-neuronal cell membranes and endoplasmic reticulum membranes such as keratinocytes, epithelial cells, and macrophages. Studies have also found that TRPV1 is also expressed in multiple regions of the central nervous system, such as the hypothalamus, striatum, and amygdala, and plays an important role in the transformation and regulation of pain in the brain. In addition, TRPV1 is also distributed in the pancreas, liver, kidneys and other organs of the human body. The widespread distribution of TRPV1 suggests that it can regulate the cell function of multiple systems and multiple organs.
The biological characteristics and physiological functions of TRPV1
In 1997, Caterina and others successfully cloned the TRPV1 receptor, which is the first cloned channel protein composed of 432 amino acids in the TRPV subfamily, and it is also one of the most studied TRP family members. TRPV1 is mainly expressed in small neurons in the dorsal root ganglia and trigeminal ganglia. However, recent studies have shown that it is also distributed in epithelium, epidermis and keratinocytes, suggesting that it has a variety of regulation on cell function. Capsaicin and gum lipotoxin (RTX), the pungent components in pepper, can activate it, because the molecular structure of capsaicin and gum lipotoxin has a similar structure to vanillin-4-hydroxy-3-methoxybenzyl, therefore, TRPV1 is also called capsaicin receptor or vanilloid receptor subtype 1 (VR1). In addition to being sensitive to capsaicin, TRPV1 can also be activated by a variety of ligand-like substances, inflammatory mediators (such as arachidonic acid metabolites), and tissue damage stimuli. In addition, TRPV1 can also be activated by non-selective stimuli, including heat (> 43 ℃), acid (pH<5.3), changes in extracellular osmotic pressure, reduction of intracellular Ca2+, intracellular redox state, electrostatic charge, etc.. This indicates that it is a multi-sensory receptor. It has been clear that the activation of TRPV1 channel protein mainly causes the influx of Ca2+ and other cations, and regulates the occurrence of corresponding physiological functions or pathological mechanisms in the form of increased intracellular Ca2+ concentration. The exact mechanism of TRPV1 receptor activation has not been fully elucidated, and it may be related to the change of the spatial conformation and stability of the gated protein by the action of stimulating factors or ligands on specific amino acid residues. Studies have found that there is an interaction between the activating substances of TRPV1. Under normal pH 7.4 conditions, the threshold for thermal activation of TRPV1 is 43℃; under acidic conditions, the activation threshold shifts to low temperature; under sufficiently low pH conditions, room temperature It can promote the opening of the TRPV1 channel, which may be the main reason why the inflammatory reaction of the respiratory tract is often accompanied by pain pathology. Recent studies have also shown that nerve growth factor (NGF) can lower the threshold of the TRPV1 channel, and its mechanism is to activate phospholipase C (PLC) to produce phosphatidylinositol 3-kinase (PI3 K). The latter p85 subunit can mediate the transfer of TRPV1 from the cytoplasm to the cell membrane, suggesting that the mechanism of sensitization of TRPV1 channels such as NGF may be related to increasing the expression of TRPV1 on the cell membrane, thereby reducing the threshold of TRPV1 channels.
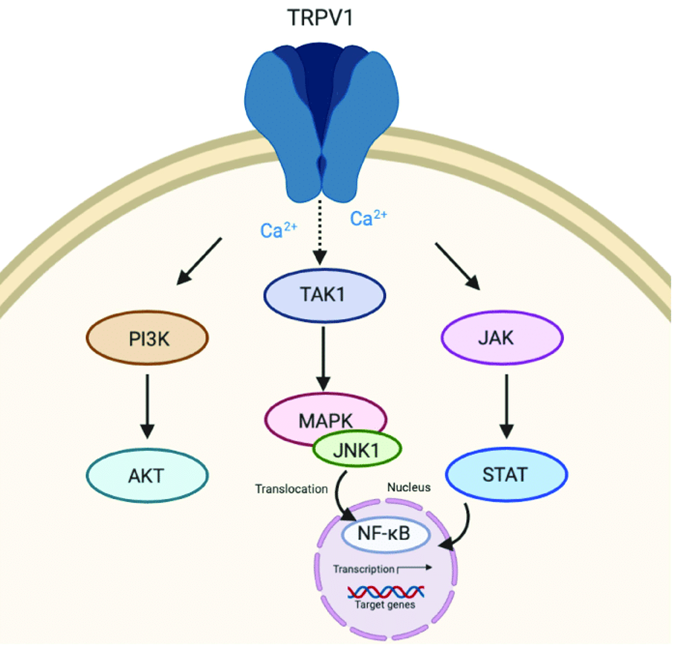
Figure 1. Transient receptor potential vanilloid 1 (TRPV1) signaling. (Nadia Peyravian, et al.; 2020)
TRPV1-mediated Pathology
TRPV1 and Pain
Pain is a common clinical symptom and is related to a variety of diseases including infection, diabetes, and nerve damage. Recent studies have shown that TRPV1 channel plays an important role in the pathogenesis of pain production and increased pain sensitivity. Bolcskei et al. injecting the PKC activator-phorbol-12-myristate-13-acetate (PMA) into the soles of mice, the mice have obvious pain defense behavior; after the TRPV1 gene is knocked out, the mice the pain defensive behavior disappeared. The former is consistent with the clinical observation that human skin is hyperalgesic to thermal and mechanical stimuli after being injured by heat. This indicates that: 1. PMA-induced pain defense behavior is specifically mediated by TRPV1; 2. TRPV1 may act as a pro-pain-causing factor in acute inflammatory pain models; 3. PKC may be involved in the formation of the pathological mechanism of post-inflammatory hyperalgesia.
TRPV1 and inflammation
In an inflammatory state, the body produces a series of chemical mediators. These inflammatory mediators can activate or sensitize nociceptors. H+ is an important pathological product in the process of inflammation. Experiments have shown that the effect of H+ on TRPV1 is sensitization rather than activation. In addition, in vivo and in vitro tests of various inflammatory mediators such as bradykinin, PGE2, extracellular ATP, glutamate, and nerve growth factor have shown that they can indirectly sensitize TRPV1 channels. Caterina et al. found that TRPV1 gene-deficient mice were irritated by mustard oil, complete Freund’s adjuvant (CFA), carrageenan, and other inflammatory mediators (bradykinin, nerve growth factor, ATP, etc.) after inflammation. Sensitivity is not significantly increased, but it is highly sensitive to mechanical stimuli; while wild mice are inflamed, the incubation period of response to heat and mechanical is significantly shortened, and the activation threshold is also significantly reduced, suggesting inflammation-induced mechanical stimuli There may be other ways or pathways for increased sensitivity. Further research found that the sensitivity of sensory nerves to heat and capsaicin increased significantly under the induction of inflammatory mediators, so that body temperature can activate TRPV1 channels. Inflammatory mediators sensitize TRPV1 mainly through the following mechanisms: 1. increase the expression of TRPV1 cytoplasmic membrane protein; 2. induce phosphorylation of TRPV1 through protein kinase pathways such as phospholipase C (PLC)-protein kinase C (PKC); 3. through 4, 5-Inositol bisphosphate pathway reduces the inhibitory effect on TRPV1. In addition, some inflammatory factors can also act on G protein-coupled receptors or activate phospholipase C (PLC) or phospholipase A2 (PLA2) through the tyrosine kinase pathway to induce the release of arachidonic acid metabolites and indirectly sensitize TRPV1 channel. In addition to inflammatory mediators, proteases released during inflammation and nerve damage, such as trypsin and mast cell tryptase, can also sensitize TRPV1 channels through second messenger pathways such as PKA and PKCε. These findings indicate that TRPV1 is not only involved in the pain transmission caused by chemical substances and thermal stimuli, but also serves as a substrate for a variety of inflammatory mediators to sensitize peripheral sensations through specific intracellular signal transduction pathways.
TRPV1 and Cough
The occurrence of cough pathology is mainly due to mechanical, physical or chemical stimulation or increased sensitivity of the receptors. Cough receptors are mainly located in the upper and lower respiratory tract (until the terminal bronchi). The sensory nerve fibers that conduct cough are located in the airway epithelium. After being stimulated, the impulse is transmitted along the sensory nerve to the cough center distributed around the respiratory center. After integration, the outgoing impulse is transmitted to the corresponding effector along the vagus nerve and other pathways. These effectors synergistic produces cough. As early as 1954, the cough model caused by inhalation of citric acid has been used to study agonists and inhibitors of the cough reflex; capsaicin has been used clinically as a test cough factor for more than 20 years, but it is difficult to analyze and study pathological features, and the exact mechanism of coughing has not been fully elucidated. Some people think that it is related to the increase of airway intraepithelial sensory neuropeptide, calmodulin gene-related peptide (CGRP) and substance P (SP); others have reported the accumulation of inflammatory mediators (such as histamine, prostaglandin, IL-6, IL- 8 etc.) sensitized cough receptors in the airway, causing cough. With the discovery and in-depth study of TRP, a superfamily of receptor ion channel proteins, people have focused their attention on the vanillin receptor subtype 1 (TRPV1) in the TRP family, and studies have found that TRPV1 channel receptor blockers -Ruthenium red (RR) and capsaicin analog-capsazepine has a significant antitussive effect on animal pathological models of cough. At present, most scholars believe that various inflammatory stimulants and exogenous stimuli activate or sensitize TRPV1 channels distributed on the sensory nerves of the respiratory tract (TRPV1 expression is upregulated, oligomerization increases, and TRPV1 migrates from the intracellular to the plasma membrane. Or phosphorylation, etc.), is the central link in the occurrence of cough.
References
- Levine JD, Nicole AH. TRP channels: targets for the relief of pain.Biochim Biophys Acta. 2007, 1772( 8) : 989-1003.
- Bourinet E, et al.; Calcium-permeable ion channels in pain signaling.Physiol Rev. 2014, 94 (1): 81-140.
- Sunitha B, et al.; Emerging roles of canonical TRP channels in neuronal function.Adv Exp Med Biol. 2011, 704: 573-593.
- BOLCSKEIK, et al.; Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005, 117 (3) 368-376.
- CHRISTOPHT, et al. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and viscer- alpain in vivo. Biochem Biophys Res Commun. 2006, 350(1) :238-243.
- CATERINAMJ, et al.; Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000, 288(5464) :306-313.
- NILIUSB, et al.; Transient receptor po- tential cation channels in disease. Physiol Rev. 2007, 87(1): 165-217.
- Nadia Peyravian, et al.; Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9: 131–140.
Inquiry
